Changes in weight and glycemic control following obesity treatment with semaglutide or tirzepatide by discontinuation status
- PMID: 40491239
- PMCID: PMC12381620
- DOI: 10.1002/oby.24331
Changes in weight and glycemic control following obesity treatment with semaglutide or tirzepatide by discontinuation status
Abstract
Objective: The objective of this study was to characterize changes in body weight and glycated hemoglobin (in those with prediabetes at baseline) through 12 months by obesity pharmacotherapy discontinuation status.
Methods: This retrospective cohort study used electronic health record data from a large health system in Ohio and Florida to identify adults with overweight or obesity without type 2 diabetes who initiated injectable semaglutide or tirzepatide between 2021 and 2023. Treatment discontinuation was defined by a >90-day gap between exhaustion of previous supply and next dispense or end of study follow-up (December 2024) and was classified into early discontinuation (i.e., within 3 months of index date) and late discontinuation (i.e., within 3-12 months).
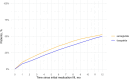
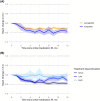
Results: We identified 7881 patients; 6109 received semaglutide, and 1772 received tirzepatide. A total of 80.8% had low maintenance dosages. Mean (SD) percentage weight reduction at 1 year was 8.7% (9.6%); and it was 3.6% (8.1%) with early discontinuation, 6.8% (9.1%) with late discontinuation, and 11.9% (9.2%) with non-discontinuation (p < 0.001). The mean (SD) absolute reduction in percent glycated hemoglobin at 1 year was 0.1 (0.4) with early discontinuation, 0.2 (0.4) with late discontinuation, and 0.4 (0.4) with non-discontinuation (p < 0.001).
Conclusions: The average weight reduction in this cohort was lower than that observed in the main phase 3 trials, likely because of higher rates of discontinuation and lower maintenance dosages.
© 2025 The Author(s). Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
W. Scott Butsch reported advisory board fees from Novo Nordisk A/S, Eli Lilly and Company, Boehringer Ingelheim, and Abbott Laboratories, as well as research funding from Eli Lilly and Company, outside the submitted work. Christopher B. Boyer reported consulting fees from Janssen Pharmaceuticals outside the submitted work. Marcio L. Griebeler reported research funding from Novo Nordisk A/S outside the submitted work. Michael B. Rothberg reported receiving consulting fees from the Blue Cross Blue Shield Association outside the submitted work. The other authors declared no conflicts of interest.
Figures

References
-
- Centers for Disease Control and Prevention . Adult obesity prevalence maps. Updated September 12, 2024. Accessed October 28, 2024. https://www.cdc.gov/obesity/data-and-statistics/adult-obesity-prevalence...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

