Revitalising Brewers' Spent Grains and Enriching With Biogenic Compounds Through the Fermentation of Fructophilic Lactic Acid Bacteria and Yeasts
- PMID: 40491241
- PMCID: PMC12149443
- DOI: 10.1111/1751-7915.70171
Revitalising Brewers' Spent Grains and Enriching With Biogenic Compounds Through the Fermentation of Fructophilic Lactic Acid Bacteria and Yeasts
Abstract
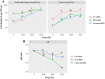
The large output of spent grains from the brewing industry presents environmental concerns but also offers promising nutritional and functional potential for valorization by researchers and industrial stakeholders. In this perspective, we investigated how non-conventional starters like Fructobacillus fructosus PL22 and Wickerhamomyces anomalus GY1 can drive the fermentation of brewer's spent grain (BSG), a solid by-product of the brewing industry, to enrich its portfolio of bioactive compounds. While sugar reduction was comparable between started- and unstarted-BSG, the effect of the fermentation became evident through the release of key microbial metabolites (lactic and acetic acids and ethanol). Both starters generated the highest number of unique peptides, with only one previously identified as antioxidant peptide found in BSG fermented with F. fructosus. During fermentation, most amino acids and phenolic compounds decreased, while BSG fermented with W. anomalus distinctly enhanced the release of Ala, Cys and GABA, and health-promoting phenolic compounds, such as gallic acid, gallocatechin, quercetin, naringenin, kaempferol, and isorhamnetin. These metabolic changes were associated with the enhanced antifungal and antioxidant properties, which in turn positively reflected on skin protection as shown by the increased proliferation of human keratinocytes, over-expression of the filaggrin (FLG) gene, and wound healing. The power of fermentation to revitalise BSG, giving it a second life chance through the improvement of its nutritional value and further multifunctionality, was demonstrated.
Keywords: antifungal activity; antioxidant activity; fructophilic lactic acid bacteria; human keratinocytes; wound healing; yeasts.
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Abeynayake, R. , Zhang S., Yang W., and Chen L.. 2022. “Development of Antioxidant Peptides From Brewers' Spent Grain Proteins.” Lwt 158: 113162. 10.1016/j.lwt.2022.113162. - DOI
-
- Almendinger, M. , Rohn S., and Pleissner D.. 2020. “Malt and Beer‐Related By‐Products as Potential Antioxidant Skin‐Lightening Agents for Cosmetics.” Sustainable Chemistry and Pharmacy 17: 100282. 10.1016/j.scp.2020.100282. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

