Integrating plasma p-tau217 and digital cognitive assessments for early detection in Alzheimer's disease
- PMID: 40491259
- PMCID: PMC12149436
- DOI: 10.1002/alz.70355
Integrating plasma p-tau217 and digital cognitive assessments for early detection in Alzheimer's disease
Abstract
Introduction: Plasma phosphorylated tau (p-tau)217 is an early Alzheimer's disease (AD) biomarker, but the timing of pathological changes and cognitive decline varies substantially. Digital cognitive assessments can detect subtle cognitive changes, suggesting they may complement p-tau217 for early detection. Here, we evaluate whether combining these tools improves the detection of individuals at risk for future decline.
Methods: We analyzed 954 amyloid-positive cognitively unimpaired individuals who completed a digital cognitive assessment and a blood test for p-tau217, assessing their ability to predict future decline on the Preclinical Alzheimer Cognitive Composite (PACC) and Mini-Mental State Examination (MMSE).
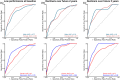
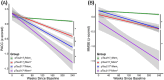
Results: Combining performance on a digital cognitive assessment with p-tau217 improved identification of individuals who declined on the PACC and MMSE in the next 5 years. The predictive value was stronger in apolipoprotein E ε4 noncarriers but did not differ by sex.
Discussion: This approach offers a sensitive method for identifying individuals at high risk for AD-related cognitive decline.
Highlights: Combining plasma phosphorylated tau 217 with baseline digital cognitive assessment improved the prediction of cognitive decline on gold-standard neuropsychological tests over the next 5 years, achieving greater accuracy than either measure alone. This combination also predicted a decline in a global cognitive screening test. Pairing a blood test with a digital cognitive assessment offers a scalable and feasible approach for Alzheimer's disease screening.
Keywords: Alzheimer's disease; digital cognitive assessments; early detection; low‐burden measures; memory; phosphorylated tau 217.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures


Update of
-
Integrating Plasma pTau-217 and Digital Cognitive Assessments for Early Detection in Alzheimer's Disease.medRxiv [Preprint]. 2025 Mar 4:2025.03.03.25323297. doi: 10.1101/2025.03.03.25323297. medRxiv. 2025. Update in: Alzheimers Dement. 2025 Jun;21(6):e70355. doi: 10.1002/alz.70355. PMID: 40093240 Free PMC article. Updated. Preprint.
References
-
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. doi: 10.1016/j.jalz.2011.03.003 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

