Soluble CD4 inhibits Ebola virus infection by targeting endosomal receptor-binding site
- PMID: 40491485
- PMCID: PMC12148371
- DOI: 10.1016/j.isci.2025.112573
Soluble CD4 inhibits Ebola virus infection by targeting endosomal receptor-binding site
Abstract
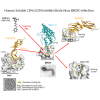
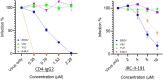
Human CD4 (cluster of differentiation 4) is well known as the primary receptor for human immunodeficiency virus (HIV) entry into the cells. The virus binds to CD4 molecules to induce a conformational change in the viral glycoprotein (GP) gp120, which exposes the co-receptor binding site for coreceptors CCR5 or CXCR4. The co-receptor binding then leads to membrane fusion for viral entry. Since the CD4 molecule has a high affinity for gp120, soluble CD4 (sCD4) and CD4-mimetic small molecules (CD4mcs) have been extensively studied as potential inhibitors for HIV infection. Surprisingly, we have found that human sCD4 and some CD4mcs are able to inhibit Ebola virus (EBOV) infection. Evidence is provided that the compounds block viral entry by targeting the GP binding site for the endosomal receptor Niemann-Pick C1 (NPC1). This finding reveals virus-receptor binding similarities between two remote viruses (HIV and EBOV) and suggests new possibilities for EBOV entry inhibitors.
Keywords: Biological sciences; Immunology; Microbiology; Natural sciences; Virology.
© 2025 The Author(s).
Conflict of interest statement
S.-H.X. and L.L.W. are inventors on US patent application no. 63/597563, on November 9, 2023, entitled “Methods and Compositions for Inhibiting Viruses”.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

