A review of conjugation technologies for antibody drug conjugates
- PMID: 40491604
- PMCID: PMC12146483
- DOI: 10.1093/abt/tbaf010
A review of conjugation technologies for antibody drug conjugates
Abstract
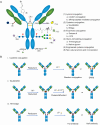
Antibody-drug conjugates (ADCs) have gained significant attention in biotherapeutics after several years of steady development. Among the multiple factors influencing ADC design, the conjugation method is one of the most critical parameters. This review classifies conjugation strategies into three categories: non-specific, site-specific but non-selective, and fully site-specific and selective methods. The characteristics; advantages and disadvantages; chemistry, manufacturing, and controls (CMC) potential; and clinical status of each conjugation strategy are discussed in detail. The site-specific and selective methods will yield more homogeneous ADC, which may influence the stability and pharmacokinetics (PK) profile of the ADC and then influence the final therapeutic outcome. Additionally, the review also explores challenges and future directions for developing novel conjugation strategies. This review presents the most prevalent conjugation techniques, providing a valuable resource for researchers in selecting conjugation technologies and advancing ADC development.
Keywords: affinity peptide conjugation; antibody–drug conjugate (ADC); cysteine conjugation; enzymatic-tag conjugation; glycan remodeling conjugation; lysine conjugation; non-canonical amino acids (ncAAs) conjugation.
© The Author(s) 2025. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
Qirui Fan, Hu Chen, Guoguang Wei, Ding Wei, Zekun Wang, Lin Zhang, and Marie Zhu are employees and stockholders of WuXi XDC Co., Ltd. Jun Wang was a former employee of WuXi XDC Co., Ltd.
Figures
References
-
- Colombo R, Tarantino P, Rich J. et al. The journey of antibody-drug conjugates: lessons learned from 40 years of development. Cancer Discov 2024;14:2089–108. 10.1158/2159-8290.CD-24-0708. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
