Low-Dose Naltrexone for Severe Fibromyalgia Syndrome: A Report of a Case With Two-Year Follow-Up
- PMID: 40491623
- PMCID: PMC12146906
- DOI: 10.7759/cureus.83824
Low-Dose Naltrexone for Severe Fibromyalgia Syndrome: A Report of a Case With Two-Year Follow-Up
Abstract
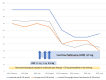
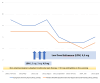
Fibromyalgia syndrome (FMS) is characterized by diffuse musculoskeletal pain associated with daytime fatigue, sleep disturbance, cognitive deficits, and often further somatic symptoms. While some patients with FMS respond to standard treatment with amitriptyline, pregabalin, or duloxetine in combination with outpatient multimodal pain management, there are still many who do not benefit sufficiently from this treatment or suffer intolerable side effects. Effective treatment options are therefore needed to supplement conventional therapies. Naltrexone is used in many countries as an off-label therapy in low doses for several chronic immunomodulatory disorders, including FMS. However, the strength of evidence from previous randomized controlled trials is low. I report a patient with severe FMS who did not respond to conventional therapy. Instead, low-dose naltrexone (LDN) (4.5 milligrams per day) resulted in a significant and sustained improvement in most FMS symptoms. The results of this case report suggest that an off-label use of LDN in severe refractory FMS may be a viable option. However, the information base is currently limited, and studies are conflicting.
Keywords: fatigue; fibromyalgia; neurology; neurophysiology; pain management; pharmacology; sleep disorder; widespread pain.
Copyright © 2025, Moser et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Low Dose Naltrexone in the Treatment of Fibromyalgia.Curr Rheumatol Rev. 2018;14(2):177-180. doi: 10.2174/1573397113666170321120329. Curr Rheumatol Rev. 2018. PMID: 28325149 Clinical Trial.
-
Low-dose naltrexone for the treatment of fibromyalgia: protocol for a double-blind, randomized, placebo-controlled trial.Trials. 2021 Nov 15;22(1):804. doi: 10.1186/s13063-021-05776-7. Trials. 2021. PMID: 34781989 Free PMC article.
-
Study protocol for a randomised, double-blinded, placebo-controlled phase III trial examining the add-on efficacy, cost-utility and neurobiological effects of low-dose naltrexone (LDN) in patients with fibromyalgia (INNOVA study).BMJ Open. 2022 Jan 6;12(1):e055351. doi: 10.1136/bmjopen-2021-055351. BMJ Open. 2022. PMID: 34992118 Free PMC article.
-
The Safety and Efficacy of Low-Dose Naltrexone in Patients with Fibromyalgia: A Systematic Review.J Pain Res. 2023 Mar 21;16:1017-1023. doi: 10.2147/JPR.S395457. eCollection 2023. J Pain Res. 2023. PMID: 36974308 Free PMC article. Review.
-
Pregabalin: its efficacy, safety and tolerability profile in fibromyalgia syndrome.Drugs Today (Barc). 2007 Dec;43(12):857-63. doi: 10.1358/dot.2007.43.12.1140689. Drugs Today (Barc). 2007. PMID: 18174971 Review.
References
-
- Microstructural evidence of neuroinflammation for psychological symptoms and pain in patients with fibromyalgia. Lo YC, Li TJ, Lin TC, Chen YY, Kang JH. J Rheumatol. 2022;49:942–947. - PubMed
-
- Neuronal toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. Leow-Dyke S, Allen C, Denes A, et al. https://doi.org/10.1186/1742-2094-9-230. J Neuroinflammation. 2012;9:230. - PMC - PubMed
-
- Nociplastic pain concept, a mechanistic basis for pragmatic approach to fibromyalgia. Bidari A, Ghavidel-Parsa B. Clin Rheumatol. 2022;41:2939–2947. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
