Green synthesis of ultrathin WS2 nanosheets for efficient hydrogen evolution reaction
- PMID: 40491805
- PMCID: PMC12146689
- DOI: 10.1039/d5ra00712g
Green synthesis of ultrathin WS2 nanosheets for efficient hydrogen evolution reaction
Abstract
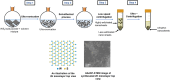
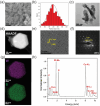
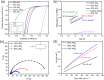
Transition metal dichalcogenide (TMD) nanostructures have emerged as promising electrocatalysts for the hydrogen evolution reaction (HER) as there is an increasing demand for cost-effective and sustainable hydrogen production. Despite significant progress, there is still a critical need for developing facile and green methods for synthesizing ultrathin TMD nanostructures. In this study, we introduce a green, top-down synthesis method to produce highly exfoliated WS2 nanosheets. The process combines the ultrasonication of bulk WS2 in a binary water-ethanol solvent with a solvothermal treatment. The resulting ultrathin WS2 nanosheets exhibit clean surfaces free of surface ligands and impurities, high crystallinity in the semiconducting hexagonal phase, and outstanding electrochemical activity for HER. Key performance metrics include a low onset potential of -0.32 V (vs. reversible hydrogen electrode (RHE)) at 10 mA cm-2 and a low Tafel slope of 160 mV dec-1 with a catalyst loading of 0.76 mg cm-2. The promising HER performance is attributed to (1) a high density of exposed edges and defects, (2) enhanced charge transport due to high crystallinity, and (3) clean surfaces enabling efficient interfacial electron transfer. Furthermore, operando Raman spectroscopy using a 3D-printed electrochemical cell identifies the catalytically active sites on WS2 nanosheets for HER. This work provides a green route to high-performance, low-dimensional electrocatalysts for sustainable hydrogen production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Vertically Aligned Ultrathin 1T-WS2 Nanosheets Enhanced the Electrocatalytic Hydrogen Evolution.Nanoscale Res Lett. 2018 May 31;13(1):167. doi: 10.1186/s11671-018-2570-x. Nanoscale Res Lett. 2018. PMID: 29855822 Free PMC article.
-
A vertical-oriented WS2 nanosheet sensitized by graphene: an advanced electrocatalyst for hydrogen evolution reaction.Nanoscale. 2015 Sep 21;7(35):14760-5. doi: 10.1039/c5nr03704b. Epub 2015 Aug 19. Nanoscale. 2015. PMID: 26287333
-
Hierarchical Fe-doped Ni3Se4 ultrathin nanosheets as an efficient electrocatalyst for oxygen evolution reaction.Nanoscale. 2018 Mar 15;10(11):5163-5170. doi: 10.1039/c8nr00426a. Nanoscale. 2018. PMID: 29492488
-
The effects of exfoliation, organic solvents and anodic activation on the catalytic hydrogen evolution reaction of tungsten disulfide.Nanoscale. 2017 Sep 21;9(36):13515-13526. doi: 10.1039/c7nr04790h. Nanoscale. 2017. PMID: 28869262
-
Preparation of High-Percentage 1T-Phase Transition Metal Dichalcogenide Nanodots for Electrochemical Hydrogen Evolution.Adv Mater. 2018 Mar;30(9). doi: 10.1002/adma.201705509. Epub 2018 Jan 15. Adv Mater. 2018. PMID: 29333655
References
-
- Coogan Á. Gun'Ko Y. K. Solution-based “bottom-up” synthesis of group VI transition metal dichalcogenides and their applications. Mater. Adv. 2021;2(1):146–164. doi: 10.1039/d0ma00697a. - DOI
-
- Han S. A. Bhatia R. Kim S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015;2:1–14. doi: 10.1186/s40580-015-0048-4. - DOI
-
- Jin H. Song T. Paik U. Qiao S.-Z. Metastable Two-Dimensional Materials for Electrocatalytic Energy Conversions. Acc. Mater. Res. 2021;2:559–573. doi: 10.1021/accountsmr.1c00115. - DOI
-
- Kwak I. H. Kwon I. S. Lee J. H. Lim Y. R. Park J. Chalcogen-vacancy group VI transition metal dichalcogenide nanosheets for electrochemical and photoelectrochemical hydrogen evolution. J. Mater. Chem. C. 2021;9(1):101–109. doi: 10.1039/d0tc04715e. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

