This is a preprint.
Convergent large-scale network and local vulnerabilities underlie brain atrophy across Parkinson's disease stages: a worldwide ENIGMA study
- PMID: 40492073
- PMCID: PMC12148252
- DOI: 10.1101/2025.05.25.25326586
Convergent large-scale network and local vulnerabilities underlie brain atrophy across Parkinson's disease stages: a worldwide ENIGMA study
Abstract
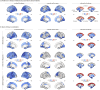
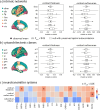
Parkinson's disease (PD) is associated with extensive structural brain changes. Recent work has proposed that the spatial pattern of disease pathology is shaped by both network spread and local vulnerability. However, only few studies assessed these biological frameworks in large patient samples across disease stages. Analyzing the largest imaging cohort in PD to date (N = 3,096 patients), we investigated the roles of network architecture and local brain features by relating regional abnormality maps to normative profiles of connectivity, intrinsic networks, cytoarchitectonics, neurotransmitter receptor densities, and gene expression. We found widespread cortical and subcortical atrophy in PD to be associated with advancing disease stage, longer time since diagnosis, and poorer global cognition. Structural brain connectivity best explained cortical atrophy patterns in PD and across disease stages. These patterns were robust among individual patients. The precuneus, lateral temporal cortex, and amygdala were identified as likely network-based epicentres, with high convergence across disease stages. Individual epicentres varied significantly among patients, yet they consistently localized to the default mode and limbic networks. Furthermore, we showed that regional overexpression of genes implicated in synaptic structure and signalling conferred increased susceptibility to brain atrophy in PD. In summary, this study demonstrates in a well-powered sample that structural brain abnormalities in PD across disease stages and within individual patients are influenced by both network spread and local vulnerability.
Keywords: Parkinson’s disease; connectivity; imaging transcriptomics; neurodegeneration; structural MRI.
Conflict of interest statement
Competing Interests M.H. currently receives payment for Advisory Board attendance/consultancy from Helicon, NeuHealth Digital, and Manus Neurodynamica. Her previous consultancies include: Lundbeck, ESCAPE Bio, Evidera, Biogen MA, CuraSen Therapeutics, Roche Products Ltd, Jazz Pharma, Aventis Pharma. K.L.P. has been on the Scientific Advisory Board for Amprion, and consults for Novartis, Lilly, BioArctic, Biohaven, Curasen and Neuron23. All other authors declare no competing interests related to this article.
Figures


References
-
- Laansma Max A., Bright Joanna K., Al-Bachari Sarah, Anderson Tim J., Ard Tyler, Assogna Francesca, Baquero Katherine A., Berendse Henk W., Blair Jamie, Cendes Fernando, Dalrymple-Alford John C., Bie Rob M.A., Debove Ines, Dirkx Michiel F., Druzgal Jason, Emsley Hedley C.A., Garraux Gäetan, Guimarães Rachel P., Gutman Boris A., Helmich Rick C., Klein Johannes C., Mackay Clare E., McMillan Corey T., Melzer Tracy R., Parkes Laura M., Piras Fabrizio, Pitcher Toni L., Poston Kathleen L., Rango Mario, Ribeiro Letícia F., Rocha Cristiane S., Rummel Christian, Santos Lucas S.R., Schmidt Reinhold, Schwingenschuh Petra, Spalletta Gianfranco, Squarcina Letizia, Heuvel Odile A., Vriend Chris, Wang Jiun-Jie, Weintraub Daniel, Wiest Roland, Yasuda Clarissa L., Jahanshad Neda, Thompson Paul M., Werf Ysbrand D., and The ENIGMA-Parkinson’s Study. International multicenter analysis of brain structure across clinical stages of parkinson’s disease. Movement Disorders, 36(11):2583–2594, January 2021. - PMC - PubMed
-
- Mak Elijah, Su Li, Williams Guy B., Firbank Michael J., Lawson Rachael A., Yarnall Alison J., Duncan Gordon W., Owen Adrian M., Khoo Tien K., Brooks David J., Rowe James B., Barker Roger A., Burn David J., and O’Brien John T.. Baseline and longitudinal grey matter changes in newly diagnosed parkinson’s disease: ICICLE-PD study. Brain, 138(10):2974–2986, 2015. - PMC - PubMed
-
- Braak Heiko, Tredici Kelly Del, Rüb Udo, de Vos Rob A.I, Jansen Steur Ernst N.H, and Braak Eva. Staging of brain pathology related to sporadic parkinson’s disease. Neurobiology of Aging, 24(2):197–211, 2003. - PubMed
-
- Braak Heiko, Udo Rüb, and Tredici Kelly Del. Cognitive decline correlates with neuropathological stage in parkinson’s disease. Journal of the Neurological Sciences, 248(1–2):255–258, October 2006. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
