This is a preprint.
β-Amyloid as a new target to suppress tonic PTH hypersecretion in primary hyperparathyroidism
- PMID: 40492090
- PMCID: PMC12148266
- DOI: 10.1101/2025.05.27.25328314
β-Amyloid as a new target to suppress tonic PTH hypersecretion in primary hyperparathyroidism
Abstract
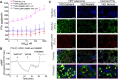
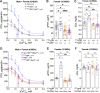
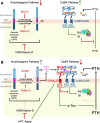
Primary hyperparathyroidism (PHPT) is a common endocrine disorder of aging closely linked to vitamin D deficiency. Reduced vitamin D receptor activities promote parathyroid hormone (PTH) hypersecretion by increasing the heterodimerization of the type B γ-aminobutyric acid receptor 1 (GABAB1R) with the extracellular Ca2+-sensing receptor (CaSR) in parathyroid cells; however, endogenous activators of the heterodimers are unknown. Here we uncovered increased expression of the β-amyloid peptide (Aβ42) cleaved from the amyloid precursor protein in parathyroid cells from PHPT patients and aging mice, and the ability of exogenous Aβ42 to promote tonic PTH secretion from murine or human parathyroid glands ex vivo. Conversely, parathyroid-specific App gene knockout reduced tonic PTH secretion and lowered serum PTH levels in mice. The absence of an Aβ42 effect on PTH secretion in parathyroid glands lacking CaSR or GABAB1R supports direct interactions between Aβ42 and the heterodimer. In situ proteomic profiling of parathyroid glands from PHPT patients closely correlated lower serum 25-hydroxyvitamin D levels with increased GABAB1R /CaSR heterodimer expression, β-amyloidogenesis, and phosphorylation of Tau, a downstream effector of Aβ42. Concurrent ablation of App or the Tau-encoding Mapt gene prevented tonic PTH hypersecretion in parathyroid-specific Vdr-KO mice. Likewise, weekly administration of an Aβ42-neutralizing antibody suppressed tonic PTH hypersecretion and synergized with daily administration of cinacalcet, a calcimimetic that activates CaSR homodimers, to reduce serum PTH levels in aging-induced hyperparathyroidism (HPT) mice. These data demonstrated novel functions of Aβ42 in driving tonic PTH secretion by activating GABAB1R/CaSR heterodimers and suggest the potential for targeting Aβ42 in PHPT treatment.
Conflict of interest statement
Competing interests: JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by Novo Nordisk, Astra Zeneca and Eli Lilly. Institutional research funding was received from Exelixis and Eli Lilly.
Figures





Similar articles
-
The efficacy and safety of cinacalcet in primary hyperparathyroidism: a systematic review and meta-analysis of randomized controlled trials and cohort studies.Rev Endocr Metab Disord. 2022 Jun;23(3):485-501. doi: 10.1007/s11154-021-09694-6. Epub 2022 Jan 18. Rev Endocr Metab Disord. 2022. PMID: 35041148
-
Parathyroidectomy for adults with primary hyperparathyroidism.Cochrane Database Syst Rev. 2023 Mar 8;3(3):CD013035. doi: 10.1002/14651858.CD013035.pub2. Cochrane Database Syst Rev. 2023. PMID: 36883976 Free PMC article.
-
Serum Parathyroid Hormone Responses to Vitamin D Supplementation in Overweight/Obese Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.Nutrients. 2017 Mar 6;9(3):241. doi: 10.3390/nu9030241. Nutrients. 2017. PMID: 28272298 Free PMC article.
-
Primary Hyperparathyroidism: A Case Series, Patient-Centered Approach to Diagnosis and Management Review.Ann Ital Chir. 2025 Jul 10;96(7):878-893. doi: 10.62713/aic.3939. Ann Ital Chir. 2025. PMID: 40665948 Review.
-
Biased antibodies and beyond: a new era in the diagnosis of PTH-dependent hypercalcemia.Endocr J. 2025 Sep 5;72(9):967-978. doi: 10.1507/endocrj.EJ25-0051. Epub 2025 May 3. Endocr J. 2025. PMID: 40335290 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
