Continuous Glucose Monitoring in the Intensive Care Unit: A Multicenter, Retrospective Hospital-Based Analysis
- PMID: 40492314
- PMCID: PMC12152008
- DOI: 10.1177/19322968251343108
Continuous Glucose Monitoring in the Intensive Care Unit: A Multicenter, Retrospective Hospital-Based Analysis
Abstract
Background: There is limited experience with continuous glucose monitoring (CGM) in intensive care units (ICUs). This study examined CGM accuracy and changes during hemodynamic instability in ICU patients with COVID-19.
Methods: We pooled data from three ICUs using CGM within a hybrid protocol combining point-of-care (POC) blood glucose testing with intermittent nonadjunctive CGM use. We compared sensor-meter agreement during lowest oxygen saturation, arterial partial pressure of oxygen (PaO2), pH, or mean arterial pressure (MAP). Linear mixed models (LMM) were used to estimate the effects of clinical condition on estimates of sensor accuracy.
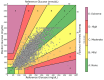
Result: Of 169 patients, >80% had a history of diabetes, mean age was 61 ± 12 years, and 82%, 93%, and 62% received corticosteroids, mechanical ventilation and vasopressors respectively. The median percent CGM time in range (TIR, 70-180 mg/dL) was 72% (64.9-81.4), 70% (54.0-76.9), and 46% (26.6-68.5) for hospitals A, B, and C. Median time below 70 mg/dL was <0.1% for all hospitals. The absolute relative difference between CGM and POC pairs did not correlate with the lowest PaO2, oxygen saturation, pH, or mean arterial pressure. In LMM adjusting for within subject and between subject variability, patients on dialysis had higher mean absolute relative difference (MARD, [coefficient = 2.39, P = .05]), while patients on mechanical ventilation had lower MARD ventilation (coefficient = -4.33, P = .05). Of the 6783 pairs 97.3% fell within Clare zones A and B.
Conclusion: These preliminary findings suggest CGM use does not appear to be significantly affected during critical illness. Confirmatory accuracy studies are needed.
Keywords: continuous glucose monitoring (CGM); critical care; hospital; inpatient; intensive care unit.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KMD discloses research support from Abbott, Insulet, and Dexcom, consulting with Eli Lilly, Insulet, Dexcom, and Oppenheimer, honorarium from Elsevier, Med Learning Group, Medscape, Impact Education, and Cardiometabolic Health Congress and royalties from UpToDate. At time of publication, JM is employed by Medtronic; he reports past research support from Dexcom and has previously served as an advisor for Medtronic Diabetes and MannKind, inc. ERF discloses research support from Dexcom and Insulet, consulting from Dexcom, and honorarium from Dexcom and Medscape. FJP discloses unrestricted research support from Insulet, Dexcom, Tandem Diabetes Care, Novo Nordisk, and Ideal Medical Technologies, personal fees from Dexcom for consulting activities, and fees to his institution from Insulet for consulting activities. JCH, YB, RB, SC, AC, MG, KP, AB, DJK, JP, NR, RS, LGJ, and MCE have no conflicts of interest to report.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

