A Novel Class of Multi-substituted Diaryl Scaffold Derivatives Inhibit Glioblastoma Progression by Targeting CD155
- PMID: 40492418
- PMCID: PMC12407320
- DOI: 10.1002/advs.202506688
A Novel Class of Multi-substituted Diaryl Scaffold Derivatives Inhibit Glioblastoma Progression by Targeting CD155
Abstract
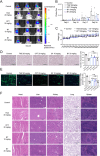
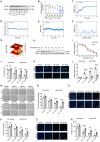
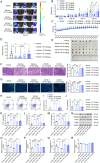
Glioblastoma (GBM) is the most formidable malignancy in the brain, characterized by a significant resistance to treatment. The immune targeting of glioblastoma stem cells (GSCs) holds great promise. In this study, structural modifications of the lead compound clofoctol is conducted and structure-activity relationship analyses are performed against GBM, yielding a novel blood-brain barrier-permeable compound, B7, featuring a pivotal multi-substituted diaryl scaffold. B7 demonstrates potent anti-GBM effects, significantly inhibiting GSC proliferation, migration, and invasion. Notably, B7 inhibits tumor progression, specifically bolstered natural killer (NK) cell-mediated cytotoxicity, and mitigates the immunosuppressive microenvironment in intracranial xenograft mice implanted with GBM cells and GSCs, as well as in cocultures of GSCs and NK-92 cells. Mechanistically, these anti-GBM effects of B7 are abolished by overexpression of poliovirus receptor cell adhesion molecule (CD155), both in vitro and in vivo. Further exploration reveals that B7 targets CD155 via interaction at five crucial binding sites, namely, L47, L108, L142, M110, and V115 residues. These interactions collectively contribute to the hydrophobic interaction energies within the B7-CD155 complex, modulating the CD155/T cell immunoreceptor with Ig and ITIM domains/CD226 axis to reshape the NK cell-mediated tumor immune microenvironment. In conclusion, this study establishes the therapeutic potential of B7 for glioma, synergistically targeting GSC biology and NK cell immunity for the treatment of GBM.
Keywords: glioblastoma stem cells; multi‐substituted diaryl derivatives; poliovirus receptor cell adhesion molecule (CD155); tumor immune microenvironment.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J. C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., French P., Hegi M. E., Jakola A. S., Platten M., Roth P., Rudà R., Short S., Smits M., Taphoorn M. J. B., von Deimling A., Westphal M., Soffietti R., Reifenberger G., Wick W., Nat. Rev. Clin. Oncol. 2021, 18, 170. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
