Lactate-Activated GPR132-Src Signal Induces Macrophage Senescence and Aggravates Atherosclerosis Under Diabetes
- PMID: 40492515
- PMCID: PMC12412570
- DOI: 10.1002/advs.202500141
Lactate-Activated GPR132-Src Signal Induces Macrophage Senescence and Aggravates Atherosclerosis Under Diabetes
Abstract
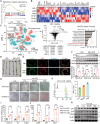
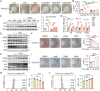
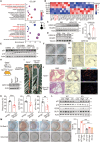
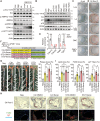
Diabetes is widely acknowledged as a significant risk factor for atherosclerosis, facilitating plaque formation through various mechanisms. Although both conditions are linked to the aging process, the relationship among cellular senescence, diabetes, and atherosclerosis remains inadequately understood. This study presents evidence that elevated glucose levels expedite the progression of atherosclerosis by promoting macrophage senescence. Increased glucose levels are shown to induce senescence in macrophages, which enhances the uptake of oxidized low-density lipoprotein (ox-LDL) and facilitates the formation of foam cells. This mechanism is driven by lactate production via glycolysis, which activates the lactate receptor GPR132, thereby promoting macrophage senescence. The activation of GPR132 is implicated in mediating senescence and lipid uptake through Src phosphorylation. The deletion of GPR132 markedly reduces macrophage senescence and atherosclerosis in mouse models. Furthermore, saracatinib, a specific Src inhibitor, has been demonstrated to effectively alleviate diabetic atherosclerosis in experimental settings. In clinical samples, elevated plasma lactate levels and the activation of the GPR132-Src pathway in peripheral blood mononuclear cells (PBMCs) are positively associated with coronary stenosis. These findings propose a potential mechanism through which diabetes accelerates atherosclerosis via the lactate-GPR132-Src pathway, underscoring macrophage senescence as a pivotal target in the context of diabetic atherosclerosis.
Keywords: atherosclerosis; diabetes; lactate; macrophage; senescence.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Libby P., Nature 2021, 592, 524. - PubMed
-
- a) Chao M. L., Luo S., Zhang C., Zhou X., Zhou M., Wang J., Kong C., Chen J., Lin Z., Tang X., Sun S., Tang X., Chen H., Wang H., Wang D., Sun J. P., Han Y., Xie L., Ji Y., Nat. Commun. 2021, 12, 4452; - PMC - PubMed
- b) Beckman J. A., Creager M. A., Libby P., JAMA, J. Am. Med. Assoc. 2002, 287, 2570. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
