Development of a novel pseudovirus-based quality control material for HIV-1 nucleic acid testing and its application in external quality assessment
- PMID: 40492710
- PMCID: PMC12210937
- DOI: 10.1128/spectrum.00269-25
Development of a novel pseudovirus-based quality control material for HIV-1 nucleic acid testing and its application in external quality assessment
Abstract
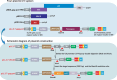
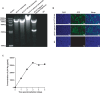
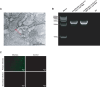
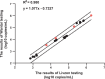
With the widespread application of HIV-1 nucleic acid testing (NAT) in China, particularly in the diagnosis of HIV-1 infection, ensuring the accuracy of NAT results through quality control has become critically important. However, existing HIV-1 NAT quality control materials (QCMs), such as clinical plasma samples and inactivated HIV-1 cell culture supernatants, have limitations in sustainability, biosafety risks, and costs. MS2-armed RNA (MS2) does not replicate the biological characteristics of natural viruses or the complexities of the extraction and detection processes associated with authentic viral particles. To address these limitations, this study developed a novel HIV-1 NAT QCM based on HIV-1 pseudovirus (PsV). HIV-1 PsV packaged using an improved four-plasmid lentiviral vector (LV) system could be generated with a high concentration of up to 10⁹ copies/mL. The HIV-1 PsV mimics the morphology of the real virus and is capable of only single-cycle infection, thereby ensuring biosafety. The HIV-1 PsV-based QCM demonstrated excellent homogeneity, absence of matrix effects, stability for 7 days at 4°C and -20°C, and the ability to withstand up to five freeze-thaw times. We further found that HIV-1 PsV outperformed inactivated HIV-1 and MS2 in terms of short-term stability and freeze-thaw stability, respectively. Additionally, the PsV-based QCM was successfully detected by 12 commercial HIV-1 NAT quantification kits in the Chinese market and demonstrated excellent performance in an external quality assessment (EQA) involving 60 laboratories. In summary, the novel HIV-1 PsV-based QCM can serve as a safe and sustainable alternative to existing HIV-1 NAT QCMs for EQA of HIV-1 NAT laboratories.IMPORTANCEThis study proposes a novel strategy to prepare HIV-1 nucleic acid testing (NAT) quality control material (QCM) using HIV-1 pseudovirus (PsV) packaged by an improved four-plasmid lentiviral vector (LV) system. The HIV-1 PsV-based QCM can simulate authentic virus particles and better monitor the entire HIV-1 NAT process, including nucleic acid extraction, amplification, and detection. The innovative HIV-1 NAT QCM possesses several desirable characteristics: biosafety, homogeneity, stability, and the ability to be prepared at high concentrations and on a large scale, significantly reducing production costs. Compared to commonly used QCMs such as inactivated HIV-1 and MS2, the HIV-1 PsV demonstrates superior stability and better meets the requirements for transportation, storage, and quality control applications of HIV-1 NAT laboratory. Particularly, the ability of HIV-1 PsV to accommodate the insertion of large nucleic acid sequences provides a solid technical foundation for developing more advanced quality control solutions in the future.
Keywords: HIV-1 nucleic acid testing; external quality assessment; lentiviral vector; pseudovirus; quality control material.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chinese Center for Disease Control and Prevention . National guideline for detection of HIV/AIDS. Available from: https://www.chinaaids.cn/fzyw_10256/jsgf/201608/W020171101497393625088
-
- National medical products administration. Available from: https://www.nmpa.gov.cn. Retrieved 27 Mar 2025.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

