Dynamics of amylopectin granule accumulation during the course of chronic Toxoplasma infection is linked to intra-cyst bradyzoite replication
- PMID: 40492719
- PMCID: PMC12306163
- DOI: 10.1128/msphere.00205-25
Dynamics of amylopectin granule accumulation during the course of chronic Toxoplasma infection is linked to intra-cyst bradyzoite replication
Abstract
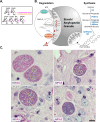
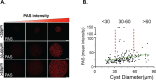
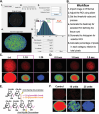
The contribution of amylopectin granules (AG), a branched chain storage homopolymer of glucose, to the maintenance and progression of the chronic Toxoplasma gondii infection has remained undefined. Here, we describe the role of AG in the physiology of encysted bradyzoites using a purpose-developed imaging-based application, AmyloQuant, which permitted the quantification of relative levels of AG within in vivo-derived tissue cysts during the initiation and maturation of chronic infection. Our findings establish that AG are dynamic, exhibiting considerable heterogeneity among tissue cysts at all post-infection time points examined. Quantification of relative steady-state AG levels within tissue cysts reveals a previously unrecognized temporal cycle involving both phases of AG accumulation and utilization over the first 6 weeks of the chronic infection. This AG cycle is temporally coordinated with overall bradyzoite mitochondrial activity. In addition, the staging of AG levels is defined by a period of low accumulation, leading into a phase of high accumulation, followed by apparent rapid utilization associated with a coordinated burst of intra-cyst bradyzoite replication. These findings suggest that AG may represent a key component in the licensing of bradyzoite replication, intimately linking stored metabolic potential to the course of the chronic infection, thereby extending the impact of AG beyond the previously assigned role in transmission. These findings force a fundamental reassessment of the chronic Toxoplasma infection, highlighting the critical need to address the temporal progression of this crucial stage in the parasite life cycle.IMPORTANCEAmylopectin granules (AG) represent a storage polymer of glucose within Toxoplasma gondii bradyzoites, the life cycle stage associated with the chronic infection. In this study, we report on the development of AmyloQuant, an image-based application, to investigate the levels and distribution of AG within encysted bradyzoites in the murine brain with the progression of the chronic infection. Quantification reveals that AG, although heterogeneous both within and across tissue cysts, exhibit a previously unrecognized temporal cycle that is linked to the overall mitochondrial activity and the capacity to replicate in vivo. This confirms that encysted bradyzoites, long considered dormant, retain considerable metabolic activity, with AG playing a potentially critical role in defining and perhaps licensing the progression of this life-long persistent infection.
Keywords: Toxoplasma gondii; amylopectin; bradyzoite; chronic infection; mitochondrial metabolism; restriction checkpoint.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




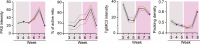
Update of
-
Dynamics of amylopectin granule accumulation during the course of the chronic Toxoplasma infection is linked to intra-cyst bradyzoite replication.bioRxiv [Preprint]. 2024 Oct 31:2024.09.02.610794. doi: 10.1101/2024.09.02.610794. bioRxiv. 2024. Update in: mSphere. 2025 Jul 29;10(7):e0020525. doi: 10.1128/msphere.00205-25. PMID: 39282379 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

