The furin cleavage site is required for pathogenesis, but not transmission, of SARS-CoV-2
- PMID: 40492735
- PMCID: PMC12282137
- DOI: 10.1128/jvi.00467-25
The furin cleavage site is required for pathogenesis, but not transmission, of SARS-CoV-2
Abstract
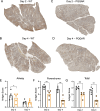
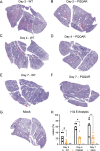
The SARS-CoV-2 spike, key to viral entry, has two features that differentiate it from other sarbecoviruses: the presence of a furin cleavage site (FCS; PRRAR sequence) and an extended S1/S2 loop characterized by an upstream QTQTN amino acid motif. Our prior works show that shortening the S1/S2 loop by deleting either the FCS (ΔPRRA) or an upstream sequence (ΔQTQTN) ablates spike processing, alters host protease usage, and attenuates infection in vitro and in vivo. With the importance of the loop length established, we evaluated the impact of disrupting the FCS while preserving the S1/S2 loop length. Using reverse genetics, we generated a SARS-CoV-2 mutant that disrupts the FCS (PQQAR) but maintains its extended S1/S2 loop. The SARS-CoV-2 PQQAR mutant has reduced replication, decreased spike processing, and attenuated disease in vivo compared to wild-type SARS-CoV-2. These data, similar to those from the FCS deletion mutant, indicate that loss of the furin cleavage site attenuates SARS-CoV-2 pathogenesis. Importantly, we subsequently found that the PQQAR mutant can be transmitted in the direct contact hamster model despite lacking an intact FCS. However, competition transmission showed that the mutant was attenuated compared to WT SARS-CoV-2. Together, the data suggest that the FCS is required for SARS-CoV-2 pathogenesis but is not strictly required for viral transmission.
Importance: The presence of the furin cleavage site (FCS) within the spike protein of SARS-CoV-2 distinguishes it from other sarbecoviruses found in nature. While prior works have deleted the FCS, these mutant viruses also shortened the S1/S2 loop, which is known to be important for pathogenesis. This study defines the importance of the FCS in the context of the extended SARS-CoV-2 S1/S2 loop. The study finds that the FCS disruption mutant is attenuated in vitro and in vivo. Disruption of the FCS reduces spike processing and changes the usage of the host protease TMPRSS2. Importantly, while not strictly required, the FCS plays a role in SARS-CoV-2 transmission efficiency. Overall, the manuscript demonstrates the importance of the furin cleavage site for SARS-CoV-2 infection, pathogenesis, and transmission.
Keywords: QTQTN; S1/S2 Loop; SARS-CoV-2; entry; furin cleavage site; protease; spike.
Conflict of interest statement
V.D.M. has filed a patent on the reverse genetic system for SARS-CoV-2. All other authors declare no conflicts of interest.
Figures



Update of
-
The furin cleavage site is required for pathogenesis, but not transmission of SARS-CoV-2.bioRxiv [Preprint]. 2025 Mar 11:2025.03.10.642264. doi: 10.1101/2025.03.10.642264. bioRxiv. 2025. Update in: J Virol. 2025 Jul 22;99(7):e0046725. doi: 10.1128/jvi.00467-25. PMID: 40161656 Free PMC article. Updated. Preprint.
Similar articles
-
The furin cleavage site is required for pathogenesis, but not transmission of SARS-CoV-2.bioRxiv [Preprint]. 2025 Mar 11:2025.03.10.642264. doi: 10.1101/2025.03.10.642264. bioRxiv. 2025. Update in: J Virol. 2025 Jul 22;99(7):e0046725. doi: 10.1128/jvi.00467-25. PMID: 40161656 Free PMC article. Updated. Preprint.
-
QTQTN motif upstream of the furin-cleavage site plays a key role in SARS-CoV-2 infection and pathogenesis.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2205690119. doi: 10.1073/pnas.2205690119. Epub 2022 Jul 26. Proc Natl Acad Sci U S A. 2022. PMID: 35881779 Free PMC article.
-
The Role of Long-Range Non-Specific Electrostatic Interactions in Inhibiting the Pre-Fusion Proteolytic Processing of the SARS-CoV-2 S Glycoprotein by Heparin.Biomolecules. 2025 May 28;15(6):778. doi: 10.3390/biom15060778. Biomolecules. 2025. PMID: 40563419 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

