Monotherapy with antibody 1C3 partially protects Ebola virus-exposed macaques
- PMID: 40492736
- PMCID: PMC12282081
- DOI: 10.1128/jvi.00296-25
Monotherapy with antibody 1C3 partially protects Ebola virus-exposed macaques
Abstract
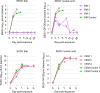
A cocktail of human monoclonal antibodies 1C3 and 1C11 previously protected macaques from a lethal exposure to either Ebola virus (EBOV) or Sudan virus (SUDV). 1C3 is of particular interest because its paratope strongly binds with unique stoichiometry to the glycoprotein head of several orthoebolaviruses, resulting in neutralization of EBOV and SUDV. Therefore, we evaluated the protective activity of 1C3 as a standalone therapeutic in macaques exposed to either EBOV or SUDV. Two doses of 1C3 monotherapy, administered 4 and 7 days post-exposure, did not protect SUDV-exposed macaques and partially protected EBOV-exposed macaques. Notably, in a macaque that succumbed to EBOV infection, we identified two mutually exclusive escape mutations that emerged immediately after the first dose and resulted in two amino acid changes at the 1C3 binding site. We also detected a subconsensus treatment-emergent mutation likely affecting the 1C3 binding site in all three deceased SUDV-exposed macaques. Our findings highlight combination treatment with 1C11 as critical for protection, particularly against SUDV, and in vivo activity of unpartnered 1C3 as susceptible to rapid EBOV and SUDV escape under therapeutic pressure.
Importance: A cocktail of human monoclonal antibodies 1C3 and 1C11 previously protected macaques exposed to a lethal dose of either Ebola virus (EBOV) or Sudan virus (SUDV). Since the unique binding characteristics of 1C3 are of particular interest, we evaluated its protective activity as monotherapy in macaques exposed to either EBOV or SUDV. Two doses of 1C3 alone did not protect SUDV-exposed macaques and only partially protected EBOV-exposed macaques. Importantly, failure to protect was associated with the rapid emergence of previously in vitro-identified escape mutations at the 1C3 binding site, highlighting the importance of its use in combination with 1C11 for protection against fatal disease outcome and avoiding rapid EBOV and SUDV escape. Findings have broader implications for the wise use of combination-based monoclonal antibody therapeutics to improve outcomes and prevent resistance in filovirid diseases.
Keywords: 1C11; 1C3; EBOV; Ebola virus; SUDV; Sudan virus; escape mutation; glycoprotein; monoclonal antibody; orthoebolavirus; partial protection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pascal KE, Dudgeon D, Trefry JC, Anantpadma M, Sakurai Y, Murin CD, Turner HL, Fairhurst J, Torres M, Rafique A, et al. 2018. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman primates. J Infect Dis 218:S612–S626. doi: 10.1093/infdis/jiy285 - DOI - PMC - PubMed
-
- Kiggundu T, Ario AR, Kadobera D, Kwesiga B, Migisha R, Makumbi I, Eurien D, Kabami Z, Kayiwa J, Lubwama B, et al. 2022. Notes from the field: outbreak of Ebola virus disease caused by sudan ebolavirus - Uganda, August-October 2022. MMWR Morb Mortal Wkly Rep 71:1457–1459. doi: 10.15585/mmwr.mm7145a5 - DOI - PMC - PubMed
-
- World Health Organization . 2025. Sudan virus disease in Uganda. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON555
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

