Toxoplasma gondii parasites induce a localized myeloid cell immune response surrounding parasites in the brain during acute infection
- PMID: 40492741
- PMCID: PMC12239593
- DOI: 10.1128/mbio.00810-25
Toxoplasma gondii parasites induce a localized myeloid cell immune response surrounding parasites in the brain during acute infection
Abstract
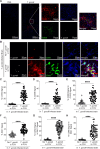
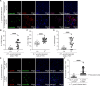
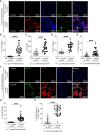
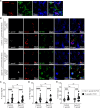
Toxoplasma gondii is a foodborne intracellular parasite that chronically infects the brain. Although T. gondii infection elicits a protective immune response, the nature of the monocyte response in the immediate vicinity of parasites during initial brain infection is not well understood. By infecting mice with T. gondii and comparing areas of the brain containing or not containing parasites, we found an increase in CCR2+ monocytes, IBA1+ myeloid cells, GFAP+ astrocytes, and CD68+ signal near parasites in the brain, indicating immune cell infiltration and phagolysosomal activation in response to the infection. CCR2+CD68+ monocytes were specifically increased near tachyzoites with minimal localization of these cells near cysts in the brain. This monocyte response was also detected near parasite-interacted cells, identified using T. gondii parasites that inject Cre recombinase into "interacted" cells of Ai6 CCR2RFP/+ mice, enabling us to track these events in vivo. The chemokine CCL2 and its transcription factor NF-κB were also upregulated surrounding parasites in the brain; however, the T. gondii effector protein GRA15, which sustains NF-κB activation in infected cells, was not required for CCL2 production, NF-κB activation, or myeloid cell recruitment to parasites in the brain. In contrast, active T. gondii replication played a more significant role, as CCR2+ monocytes were recruited to replication-competent but not replication-deficient T. gondii delivered via intracranial injection into mice. These findings provide novel insights into the drivers of immune cell mobilization and activation in the brain during initial T. gondii central nervous system infection and highlight the importance of parasite replication in this process.
Importance: Toxoplasma gondii is a brain-infecting parasite, and the mobilization of peripheral immune cells to the brain is critical for controlling T. gondii infection. However, the initial events driving these cells to sites of T. gondii infection in the brain are poorly understood. We show that peripheral myeloid immune cell recruitment and activation are specific to areas of the brain containing actively replicating parasites, which are capable of lysing host cells. This local immune response is characterized by focal chemokine production, myeloid cell recruitment, and the activation of phagolysosomal pathways. These highly localized host responses were independent of the parasite effector protein that induces NF-κB activation within infected cells, GRA15. However, the localized monocyte recruitment was dependent on live parasites and active parasite replication. This research highlights the importance of host cell sensing of parasite replication in the brain for immune control of T. gondii infection.
Keywords: Toxoplasma gondii; brain; immune response; monocyte; myeloid cells; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Injection with Toxoplasma gondii protein affects neuron health and survival.Elife. 2021 Jun 9;10:e67681. doi: 10.7554/eLife.67681. Elife. 2021. PMID: 34106047 Free PMC article.
-
Toxoplasma gondii Dissemination in the Brain Is Facilitated by Infiltrating Peripheral Immune Cells.mBio. 2022 Dec 20;13(6):e0283822. doi: 10.1128/mbio.02838-22. Epub 2022 Nov 29. mBio. 2022. PMID: 36445695 Free PMC article.
-
Endogenous IL-27 during toxoplasmosis limits early monocyte responses and their inflammatory activation by pathological T cells.mBio. 2024 Mar 13;15(3):e0008324. doi: 10.1128/mbio.00083-24. Epub 2024 Feb 20. mBio. 2024. PMID: 38376210 Free PMC article.
-
Activities of anti-Toxoplasma drugs and compounds against tissue cysts in the last three decades (1987 to 2017), a systematic review.Parasitol Res. 2018 Oct;117(10):3045-3057. doi: 10.1007/s00436-018-6027-z. Epub 2018 Aug 8. Parasitol Res. 2018. PMID: 30088074
-
Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis.Lancet HIV. 2017 Apr;4(4):e177-e188. doi: 10.1016/S2352-3018(17)30005-X. Epub 2017 Feb 1. Lancet HIV. 2017. PMID: 28159548
References
-
- Webster JP. 2010. Dubey, J.P. toxoplasmosis of animals and humans. Parasites Vectors 3:112. doi: 10.1186/1756-3305-3-112 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

