Immunogenicity of NSDV GP38 and the role of furin in GP38 proteolytic processing
- PMID: 40492757
- PMCID: PMC12282141
- DOI: 10.1128/jvi.00537-25
Immunogenicity of NSDV GP38 and the role of furin in GP38 proteolytic processing
Abstract
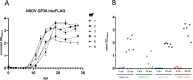
Nairobi sheep disease virus (NSDV) is a tick-borne orthonairovirus, which is genetically related to Crimean-Congo hemorrhagic fever virus (CCHFV), and causes severe hemorrhagic gastroenteritis in infected sheep. CCHFV GP38, a cleavage product of the CCHFV glycoprotein precursor (GPC), has recently attracted attention: not only has GP38 been reported to elicit detectable anti-GP38 antibodies in CCHFV-infected patients, but anti-GP38 antibodies have also been shown to protect mice from lethal CCHFV challenge. While proteolytic cleavage of CCHFV GP38 has been described to involve the proprotein convertases furin and subtilisin/kexin-isozyme-1 (SKI-1), little is known about the processing of NSDV GPC, or the occurrence and immunogenicity of NSDV GP38 in infected sheep. Here, we provide the first evidence for the presence and immunogenicity of NSDV GP38 in infected sheep demonstrating seroconversion by the detection of anti-GP38 antibodies over the course of infection. To further characterize GPC processing in vitro, we investigated the impact of furin overexpression and the effect of a furin inhibitor on NSDV glycoprotein expression, cleavage, and viral infectivity. While virus infectivity remained unaffected, our results suggest that other proteases besides furin may play a role in the proteolytic processing of NSDV GPC at a cleavage site that remains to be explored. Taken together, our findings highlight the immunogenicity of NSDV GP38 in sheep and warrant further research into the similarities and differences in proteolytic cleavage between the glycoproteins of NSDV and other orthonairoviruses, such as CCHFV.
Importance: Nairobi sheep disease virus (NSDV) is a zoonotic orthonairovirus causing severe and often fatal hemorrhagic gastroenteritis in small ruminants. Its genetic relationship to human-pathogenic Crimean-Congo hemorrhagic fever virus (CCHFV) and striking similarities in the clinical picture between CCHFV-infected patients and NSDV-infected ruminants have led to the idea that NSDV could serve as a model organism to study CCHFV pathogenesis. However, knowledge on NSDV-host interactions has been limited. While CCHFV GP38 has recently attracted attention as vaccine candidate and possible virulence factor, the occurrence and role of putative GP38 in other orthonairoviruses has been unclear. This study provides first evidence for the presence and immunogenicity of NSDV GP38 in infected sheep. Furthermore, our data indicate that other proteases besides furin may be involved in the proteolytic cleavage of NSDV GPC. Future studies are needed to determine the proteases involved and to investigate the possible functional role of GP38 in NSDV pathogenesis.
Keywords: CCHFV; GP38; NSDV; furin; orthonairovirus; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. 2017. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antiviral Res 144:93–119. doi: 10.1016/j.antiviral.2017.05.010 - DOI - PMC - PubMed
-
- Bernard C, Holzmuller P, Bah MT, Bastien M, Combes B, Jori F, Grosbois V, Vial L. 2022. Systematic review on Crimean–Congo hemorrhagic fever enzootic cycle and factors favoring virus transmission: special focus on France, an apparently free-disease area in Europe. Front Vet Sci 9:932304. doi: 10.3389/fvets.2022.932304 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

