Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis of three randomized controlled trials and review of ongoing trials
- PMID: 40493076
- PMCID: PMC12152056
- DOI: 10.1007/s00701-025-06587-4
Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis of three randomized controlled trials and review of ongoing trials
Abstract
Background: Middle Meningeal Artery Embolization (MMAE) has been proposed as adjunct and stand-alone treatment for Chronic Subdural Hematoma (CSDH). We aimed to meta-analyze three recently published randomized controlled trials, to reliably estimate the effect of MMAE. We also carried out a systematic review of ongoing trials and their key outcomes.
Methods: A PRISMA-compliant meta-analysis was conducted (PROSPERO ID CRD42024618816). Three published RCTs (MAGIC-MT, EMBOLISE, and STEM) assessing MMAE in CSDH were included. Trial primary outcomes were pooled for analysis using random effects models. Primary and secondary outcomes (recurrence/surgical rescue, functional outcome) were obtained, stratified by treatment group (undergoing surgery, and nonsurgical management). A descriptive review of trials in public registries was also conducted (search date 30th November 2024).
Results: In total, 1432 patients were included from three trials in meta-analysis. Overall, MMAE reduced symptomatic progression or recurrence, but was not statistically significant (RR 0.50, 95% CI 0.23-1.06, P = 0.058). For the group undergoing surgery, MMAE was not associated with reduced recurrence (RR 0.60, 95% CI 0.19-1.88, P = 0.194). For nonsurgical management, MMAE reduced progression (RR 0.36, 95% CI 0.22-0.60, P < 0.001). MMAE did not influence functional outcome (RR 1.01, 95% CI 0.97-1.04, P = 0.790). From the literature search, there are twenty-one registered trials. Nineteen studies include arms assessing MMAE as an adjunct to surgery, eleven compare MMAE to observation, and four with surgery. The most common primary outcome is recurrence (47.8%, N = 11), either radiologically, or requiring a second surgery. Inclusion criteria, embolization agents, primary and secondary outcomes differed significantly between studies.
Conclusions: In this meta-analysis of three randomized controlled trials, the use of MMAE in patients undergoing surgery did not appear to significantly reduce recurrence or improve functional outcomes, but did reduce progression in nonsurgical cohorts. Further studies assessing these cohorts are ongoing.
Keywords: Chronic subdural hematoma; MMAE; Middle meningeal artery embolization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate declarations: Not applicable. Competing interests: TM: Has received educational travel grants from Balt and Medtronic. AK: AK has been previously supported by NIHR (Dex-CSDH trial).
Figures


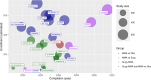
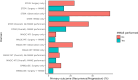
References
-
- Ban SP et al (2018) Middle meningeal artery embolization for chronic subdural hematoma. Radiology 286(3):992–999 - PubMed
-
- Brannigan JFM et al (2024) Impact of antithrombotic agents on outcomes in patients requiring surgery for chronic subdural haematoma: a systematic review and meta-analysis. Br J Neurosurg 1–8. 10.1080/02688697.2024.2333399 - PubMed
-
- Brennan PM et al (2017) The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg 127(4):732–739 - PubMed
-
- Catapano JS et al (2021) Middle meningeal artery embolization for chronic subdural hematoma: an institutional technical analysis. J Neurointerv Surg 13(7):657–660 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

