The macrophage-intrinsic MDA5/IRF5 axis drives HIV-1 intron-containing RNA-induced inflammatory responses
- PMID: 40493408
- PMCID: PMC12352897
- DOI: 10.1172/JCI187663
The macrophage-intrinsic MDA5/IRF5 axis drives HIV-1 intron-containing RNA-induced inflammatory responses
Abstract
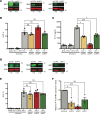
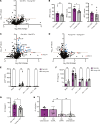
Despite effective antiretroviral therapy, transcriptionally competent HIV-1 reservoirs remain and contribute to persistent immune activation in people living with HIV (PWH). HIV-1-infected macrophages are important mediators of chronic innate immune activation, though the mechanisms remain unclear. We previously reported that nuclear export and cytoplasmic expression of HIV-1 intron-containing RNA (icRNA) activates mitochondrial antiviral signaling (MAVS) protein-mediated type I IFN responses in macrophages. In this study, we demonstrate an essential role of melanoma differentiation-associated protein 5 (MDA5) in sensing HIV-1 icRNA and promoting MAVS-dependent interferon regulatory factor 5 (IRF5) activation in macrophages. Suppression of MDA5 but not retinoic acid-inducible gene I expression nor disruption of the endosomal TLR pathway abrogated HIV-1 icRNA-induced type I IFN responses and IP-10 expression in macrophages. Furthermore, induction of IP-10 in macrophages upon HIV-1 icRNA sensing by MDA5 was dependent on IRF5. Additionally, monocytes and monocyte-derived macrophages (MDMs) from older (>50 years) individuals exhibited constitutively higher levels of IRF5 expression compared with younger (<35 years) individuals, and HIV-1 icRNA-induced IP-10 expression was significantly enhanced in older macrophages, which was attenuated upon ablation of IRF5 expression, suggesting that IRF5 functions as a major mediator of proinflammatory response downstream of MDA5-dependent HIV-1 icRNA sensing, dysregulation of which might contribute to chronic inflammation in older PWH.
Keywords: AIDS/HIV; Aging; Inflammation; Innate immunity; Macrophages.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

