B cell deficiency induces cytotoxic memory CD8+ T cells during influenza-associated bacterial pneumonia
- PMID: 40493422
- PMCID: PMC12352901
- DOI: 10.1172/JCI188342
B cell deficiency induces cytotoxic memory CD8+ T cells during influenza-associated bacterial pneumonia
Abstract
Influenza-associated bacterial superinfections in the lung lead to increased morbidity and mortality. Nearly all people have preexisting memory to influenza virus, which can protect against subsequent infection in the lung. This study explored the role B cells play in protection against bacterial (Staphylococcus aureus or Klebsiella pneumoniae) superinfection with previous heterotypic influenza memory. B cell deficiency resulted in an increased inflammatory lung environment and lung tissue injury during superinfection. Loss of B cells increased populations of memory CD8+ T cells in the lung, and these CD8+ T cells were transcriptionally and functionally distinct from those of WT mice. Use of antibody-deficient mouse models showed that this phenotype was specifically due to loss of antibody production from B cells. Passive immunization with influenza antibody serum in B cell-deficient mice rescued the CD8+ T cell phenotype. CD8+ T cell depletion and lethal superinfection challenge experiments showed that the cytotoxic memory CD8+ T cells from B cell-deficient mice protect against superinfection bacterial burden and mortality. These findings provide insight into the importance of B cells for regulating immune responses against infection.
Keywords: Adaptive immunity; Immunology; Infectious disease; Influenza; T cells.
Conflict of interest statement
Figures
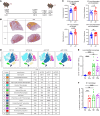


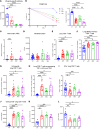
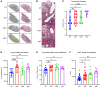

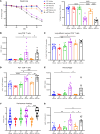

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

