Clinical study outcomes in IgA nephropathy: A systematic literature review and narrative synthesis
- PMID: 40493692
- PMCID: PMC12151485
- DOI: 10.1371/journal.pone.0323530
Clinical study outcomes in IgA nephropathy: A systematic literature review and narrative synthesis
Abstract
Introduction: IgA nephropathy (IgAN) is an inflammatory kidney disease which, if left untreated, often progresses to kidney failure (KF). This systematic literature review identifies, collates, summarizes, and assesses the quality of clinical trial data describing the efficacy of therapies used for IgAN.
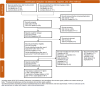
Methods: Ovid Embase, PubMed, CENTRAL, and the Cochrane database of systematic reviews were searched on October 18th, 2021, and updated on December 12th, 2023. Electronic searches were supplemented with manual searches of key conferences, clinical trial registries, and bibliography screening. PRISMA and Cochrane guidelines were followed.
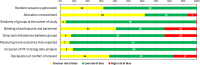
Results: A total of 6710 references were identified (electronic and manual searches), of which 6483 were excluded. This resulted in 254 references reporting 183 studies which met our inclusion criteria. The majority of these IgAN studies (98/183 studies [60%]) had a non-randomized or single-arm design and/or a small population size or focused on dietary and traditional medicine, resulting in a high risk of bias and necessitated additional filtering to prioritize larger (n>30) randomized assessment of pharmacological interventions reporting key clinical outcomes. This additional filtering resulted in 76 randomized controlled trials (100 references) selected for narrative synthesis; 60 reported proteinuria outcomes and 18 reported estimated glomerular filtration rate (eGFR) outcomes.
Conclusions: Until recently, the evidence has been mixed or inconsistent across studies for the efficacy of IgAN treatments in reducing proteinuria or slowing eGFR decline due to a high risk of bias in many included studies. The latest large, phase 3 NefIgArd (NCT03643965) and PROTECT (NCT03762850) clinical trials have demonstrated a meaningful reduction in proteinuria and eGFR decline for patients with IgAN receiving targeted-release formulation budesonide (TRF-B) or sparsentan. Results from other high-quality randomized controlled trials with a follow-up period of at least 2 years are still required to better support advancements in the management of IgAN.
Copyright: © 2025 . This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
AJ has served on a scientific advisory board for Calliditas Therapeutics. KDJ is a founder and co-president of the American Society of Onco-Nephrology; reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals and Calliditas. KDJ reports honoraria from the American Society of Nephrology, the ISN, and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. MB is a consultant for Travere Therapeutics, Inc. JAB is an employee, and DMWC was an employee, of Genesis Research Group which received compensation from Travere Therapeutics, Inc. for conducting this study. MEB is a consultant for Travere Therapeutics, Inc. and reports an additional consultancy agreement with Amgen, Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- O’Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. Am Fam Physician. 2012;85(7):705–10. - PubMed
-
- Barratt J, Saleem M, Braddon F, Carroll K, He P, Hendry B. Natural history of IgA nephropathy: analysis of a UK national RaDaR IgA nephropathy cohort. In: 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

