Cellular anatomy of arbuscular mycorrhizal fungi
- PMID: 40494310
- PMCID: PMC12165283
- DOI: 10.1016/j.cub.2025.03.053
Cellular anatomy of arbuscular mycorrhizal fungi
Abstract
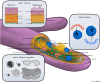
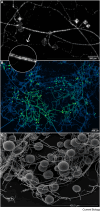
Arbuscular mycorrhizal (AM) fungi are ancient plant mutualists that are ubiquitous across terrestrial ecosystems. These fungi are unique among most eukaryotes because they form multinucleate, open-pipe mycelial networks, where nutrients, organelles, and chemical signals move bidirectionally across a continuous cytoplasm. AM fungi play a crucial role in ecosystem functioning by supporting plant growth, mediating ecosystem diversity, and contributing to carbon cycling. It is estimated that plant communities allocate ∼3.93 Gt CO2e to AM fungi every year, much of which is stored as lipids inside the fungal network. Despite their ecological significance, the cellular biology of AM fungi remains underexplored. Here, we synthesise the current knowledge on AM fungal cellular structure and organisation. We examine AM fungal development at different biological levels - the hypha and its content, hyphal networks and AM fungal spores - and explore key cellular dynamics. This includes cell wall composition, cytoplasmic contents, nuclear and lipid organisation and dynamics, network architecture, and connectivity. We highlight how their unique cellular arrangement enables complex cytoplasmic flow and nutrient exchange processes across their open-pipe mycelial networks. We discuss how both established and novel techniques, including microscopy, culturing, and high-throughput image analysis, are helping to resolve previously unknown aspects of AM fungal biology. By comparing these insights with established knowledge in other, well-studied filamentous fungi, we identify critical knowledge gaps and propose questions for future research to further our understanding of fundamental AM fungal cell biology and its contributions to ecosystem health.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Xizang meadow degradation alters resource exchange ratio, network complexity, and biomass allocation tradeoff of arbuscular mycorrhizal symbiosis.New Phytol. 2025 Feb;245(3):1288-1301. doi: 10.1111/nph.20304. Epub 2024 Nov 29. New Phytol. 2025. PMID: 39611464
-
Plant Identity Exerts Stronger Effect than Fertilization on Soil Arbuscular Mycorrhizal Fungi in a Sown Pasture.Microb Ecol. 2016 Oct;72(3):647-58. doi: 10.1007/s00248-016-0817-6. Epub 2016 Jul 16. Microb Ecol. 2016. PMID: 27423979
-
Plant functional groups associate with distinct arbuscular mycorrhizal fungal communities.New Phytol. 2020 May;226(4):1117-1128. doi: 10.1111/nph.16423. Epub 2020 Feb 17. New Phytol. 2020. PMID: 31943225
-
Ecological and evolutionary implications of hyphal anastomosis in arbuscular mycorrhizal fungi.FEMS Microbiol Ecol. 2014 Jun;88(3):437-44. doi: 10.1111/1574-6941.12321. Epub 2014 Apr 7. FEMS Microbiol Ecol. 2014. PMID: 24646134 Review.
-
Genetic processes in arbuscular mycorrhizal fungi.FEMS Microbiol Lett. 2005 Oct 15;251(2):185-92. doi: 10.1016/j.femsle.2005.08.007. FEMS Microbiol Lett. 2005. PMID: 16140474 Review.
References
-
- Soudzilovskaia N.A., Van Bodegom P.M., Terrer C., Zelfde M.V., McCallum I., McCormack M.L., Fisher J.B., Brundrett M.C., De Sá N.C., Tedersoo L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019;10:5077. doi: 10.1038/s41467-019-13019-2. - DOI - PMC - PubMed
-
- Montoliu-Nerin M., Sánchez-García M., Bergin C., Kutschera V.E., Johannesson H., Bever J.D., Rosling A. In-depth phylogenomic analysis of arbuscular mycorrhizal fungi based on a comprehensive set of de novo genome assemblies. Front. Fungal Biol. 2021;2 doi: 10.3389/ffunb.2021.716385. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

