Biopsy-proven PACNS: results from the large, multicentre cohort of cerebral vasculitis patients
- PMID: 40494617
- PMCID: PMC12421100
- DOI: 10.1136/jnnp-2025-335764
Biopsy-proven PACNS: results from the large, multicentre cohort of cerebral vasculitis patients
Abstract
Background: Reports of primary angiitis of the central nervous system (PACNS) are mainly restricted to clinically suspected cases, but biopsy-proven ones are rare. Here, we present results from a large multicentre cohort of patients with biopsy-proven PACNS (BP-PACNS). In particular, we provide insights into characteristics and treatment responses of PACNS subtypes.
Methods: BP-PACNS patients treated between 1999 and 2021 were analysed. The outcome was assessed by the modified Rankin Scale (mRS). Between-group comparisons were performed by Kruskal-Wallis, χ2 or Fisher's exact tests.
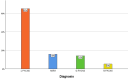
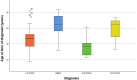
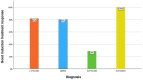
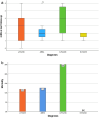
Results: In total, 57 patients were analysed (52% male). Of these, n=37 (65%) had a lymphocytic (L-PACNS), n=9 (16%) an amyloid-beta-related angiitis (ABRA), n=8 (14%) a granulomatous (G-PACNS) and n=3 (5%) a necrotising (N-PACNS) PACNS subtype. At the time of diagnosis, age differed significantly between groups (median age (years) L-PACNS 47, ABRA 64.5, G-PACNS 37, N-PACNS 65; p=0.008). The clinical course was mostly monophasic in L-PACNS and ABRA (65% and 75%, respectively), while relapsing-remitting in G-PACNS (63%). Median mRS at last follow up was 2 (IQR 1.25-4) in the study group. Worst outcome (median mRS 4) and highest mortality (25%) were seen in G-PACNS. Good induction treatment response was achieved in 77% of all BP-PACNS patients but was low in those with G-PACNS (29%).
Conclusions: In this large, multicentre series of BP-PACNS patients, G-PACNS had a worse functional outcome, a predominant relapsing-remitting disease and a poorer response to the induction therapy. An optimal first-line treatment regimen for G-PACNS patients should be further examined and established in larger studies to improve the outcome of G-PACNS patients.
Keywords: CEREBROVASCULAR DISEASE; STROKE; VASCULITIS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MD-C has received research grants from the Werner Otto Foundation, speakers honoraria from AstraZeneca and was funded by the Faculty of Medicine, University of Kiel, Germany through its Advanced Clinician Scientist Programme, all outside the submitted work. PA, HP, CK, JH, JL and HE: none. FJB received speakers honoraria from AstraZeneca, outside the submitted work. CHN received speaker honoraria for lectors from Alexion, Astra Zeneca, BMS, Novartis and Pfizer, all outside the submitted work. TM has received personal fees from Merck-Serono, Boehringer Ingelheim, Bristol-Myers Squibb/ Pfizer, Novartis, Biogen, Grifols, CSL-Behring, grants from the European Union (FP-7, ERA-NET), German Research Foundation, Herrmann and Lilly Schilling Foundation, and Werner-Otto Foundation, all outside the submitted work.
Figures
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
