Polystyrene nanoplastics disrupt the intestinal microenvironment by altering bacteria-host interactions through extracellular vesicle-delivered microRNAs
- PMID: 40494850
- PMCID: PMC12152142
- DOI: 10.1038/s41467-025-59884-y
Polystyrene nanoplastics disrupt the intestinal microenvironment by altering bacteria-host interactions through extracellular vesicle-delivered microRNAs
Abstract
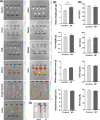
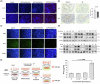
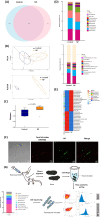
Nanoplastics (NP) are emerging environmental pollutants with potential risks to human health. This study investigates how polystyrene-NP exposure disrupts the intestinal microenvironment and barrier function through bacteria-host interactions. Using in vivo models and bacterial sorting technology, we show that NP accumulation in the mouse intestine alters the expression of intestinal miR-501-3p and miR-700-5p, compromising tight junction protein ZO-1 and mucin (MUC)-13 expression, thereby increasing intestinal permeability. NP increases miR-98-3p, miR-548z, miR-548h-3o, miR-548d-3p, miR-548az-5p, miR-12136, and miR-101-3p levels in extracellular vesicles (EVs) derived from goblet-like cells, which can interfere with ZO-1 expression. NP also induces gut microbiota dysbiosis, characterized by elevated Ruminococcaceae abundance and altered EV characteristics from goblet cells. Lachnospiraceae internalize NP, and their EVs suppress MUC-13 expression. These findings reveal a mechanism by which NP compromises intestinal integrity and indirectly alters intestinal microbiota composition, potentially leading to adverse health outcomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- MOST 110-2628-B-415-001/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)
- MOST 109-2636-B-006-008/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)
- MOHW110-FDA-F-114-000374/Ministry of Health and Welfare, Taiwan | Food and Drug Administration (Taiwan Food and Drug Administration)
LinkOut - more resources
Full Text Sources
Miscellaneous

