Deep learning-based ranking method for subgroup and predictive biomarker identification in patients
- PMID: 40494908
- PMCID: PMC12152138
- DOI: 10.1038/s43856-025-00946-z
Deep learning-based ranking method for subgroup and predictive biomarker identification in patients
Abstract
Background: The task of identifying patient subgroups with enhanced treatment responses is important for clinical drug development. However, existing deep learning-based approaches often struggle to provide clear biological insights. This study aims to develop a deep learning method that not only captures treatment effect differences among individuals but also helps uncover meaningful biological markers associated with those differences.
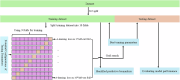
Methods: We introduce DeepRAB, a deep learning-based framework designed for exploring treatment effect heterogeneity by constructing individualized treatment rule (ITR). In addition, DeepRAB enables model interpretability by facilitating predictive biomarker identification. We evaluate its performance using simulated datasets that vary in complexity, treatment effect strength, and sample size. We also apply the method to the adalimumab (Humira, AbbVie) hidradenitis suppurativa (HS) clinical trial data, analyzing patient characteristics and treatment outcomes.
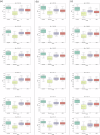
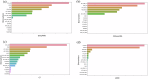
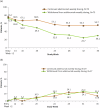
Results: In analyses of simulated data under various scenarios, our findings show the effective performance of DeepRAB for subgroup exploration, and its capability to uncover predictive biomarkers when compared to existing approaches. When applied to the real clinical trial data, DeepRAB demonstrates its practical usage in identifying important predictive biomarkers and boosting model prediction performance.
Conclusions: Our research provides a promising approach for subgroup identification and predictive biomarker discovery by leveraging deep learning. This approach may support more targeted treatment strategies in clinical research and enhance decision-making in personalized medicine.
Plain language summary
In order to improve healthcare, matching patients to effective treatment plans is needed. This study aims to find better ways to identify groups of patients who respond well to certain treatments. We develop a new method called DeepRAB, which uses artificial intelligence to find these patient groups and identify important biomarkers that can predict treatment response. We test DeepRAB using simulated data and real patient data from a clinical trial studying a skin condition called hidradenitis suppurativa. The results show that DeepRAB is successful in identifying meaningful patient groupings and performs better than existing methods. This new approach has the potential to help doctors choose the best treatment options for individual patients, making healthcare more personalized and effective.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Two Cases of Hidradenitis Suppurativa Treated with Adalimumab at the Department of Dermatology and Venereology, Clinical Hospital Mostar.Acta Dermatovenerol Croat. 2021 Jul;29(2):108-110. Acta Dermatovenerol Croat. 2021. PMID: 34477078
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Deep convolutional neural network and IoT technology for healthcare.Digit Health. 2024 Jan 17;10:20552076231220123. doi: 10.1177/20552076231220123. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 38250147 Free PMC article.
-
Using Visual Analytic Methods to Identify Patient Groups [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Jun. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Jun. PMID: 38781405 Free Books & Documents. Review.
References
-
- Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol.23, 985–998 (2009). - PubMed
-
- Jemec, G. B. Hidradenitis suppurativa. N. Engl. J. Med.366, 158–164 (2012). - PubMed
-
- Shlyankevich, J., Chen, A. J., Kim, G. E. & Kimball, A. B. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J. Am. Acad. Dermatol.71, 1144–1150 (2014). - PubMed
-
- Kimball, A. B. et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N. Engl. J. Med.375, 422–434 (2016). - PubMed
-
- Kimball, A. et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo‐controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol.30, 989–994 (2016). - PMC - PubMed
LinkOut - more resources
Full Text Sources

