T-cell responses induced by SARS-CoV-2 index-virus nanoparticle protein vaccine to the ancestral and omicron variants 6 months following primary vaccination
- PMID: 40494917
- PMCID: PMC12152139
- DOI: 10.1038/s43856-025-00941-4
T-cell responses induced by SARS-CoV-2 index-virus nanoparticle protein vaccine to the ancestral and omicron variants 6 months following primary vaccination
Abstract
Background: The SARS-CoV-2 index-virus nanoparticle protein vaccine (NVX-CoV2373) induces humoral and cell-mediated immune responses that protect against severe COVID-19, including from SARS-CoV-2 variants. Limited information exists on NVX-CoV2373-induced cell-mediated immune responses to ancestral SARS-CoV-2 and the Omicron variant following a homologous booster (third dose), and on T-cell responses following a booster dose compared to a single dose.
Methods: T-cell responses were investigated in participants from a randomised, placebo-controlled, phase 2A/2B trial of NVX-CoV2373 in South Africa, who had a blinded crossover at 6 months post-enrolment. Peripheral blood mononuclear cells were available for 34 participants, 7 days post-vaccination with one NVX-CoV2373 dose (n = 17) or a homologous booster (n = 17). T-cell responses to the full-length spike (FLS) glycoprotein of ancestral Wuhan-Hu-1 SARS-CoV-2 and mutated spike protein regions found in Omicron (BA.4/BA.5) were characterised by intracellular cytokine staining.
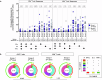
Results: Here we show that FLS-specific T-cell responses are similar between single-dose and booster-dose recipients (CD4+: p = 0.871; CD8+: p = 0.491) and are predominantly monofunctional (IFN-γ or TNF-α). A third NVX-CoV2373 dose increases the FLS-specific polyfunctional cytokine production profile of CD4+ T cells compared with after a single dose (p = 0.045), whereas CD8+ T cells remain unaffected (p = 0.462). Only CD4+ T cells exhibit reduced reactivity to Omicron compared with ancestral SARS-CoV-2 in single-dose (p = 0.010) and in booster-dose recipients (p = 0.028).
Conclusions: NVX-CoV2373-induced T-cell responses to ancestral SARS-CoV-2 are comparable following vaccination with a single dose compared with a third dose administered 6 months after the second dose. Our findings suggest that an NVX-CoV2373 booster dose does not enhance T-cell immunity. Furthermore, NVX-CoV2373 vaccination induces greater T-cell response magnitudes to ancestral SARS-CoV-2, from which the vaccine is derived, compared with the Omicron variant.
Plain language summary
The NVX-CoV2373 vaccine was the first protein-based COVID-19 vaccine to receive emergency use authorisation in the USA. It stimulates both antibody and T-cell responses that help protect against severe illness caused by the original SARS-CoV-2 virus and emerging variants. Antibodies are proteins that recognise and block the virus, preventing it from entering cells. T cells are a type of white blood cell that help identify and eliminate virus-infected cells. We studied T-cell responses in people with no prior SARS-CoV-2 infection who received either one or three NVX-CoV2373 doses. We found that a third dose did not further increase T-cell responses. T-cell responses were also stronger to the original SARS-CoV-2 virus than to the Omicron variant. These findings highlight the need for improved vaccines and tailored vaccination strategies to effectively combat evolving SARS-CoV-2 variants.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: S.A.M., M.C.N. and G.K. report receiving grant support, paid to their institution, from Novavax and the Bill & Melinda Gates Foundation; V.S. and C.B. report being employed by and owning shares in Novavax and contributed to the interpretation of the study’s results. No competing interests were declared for the remaining authors.
Figures


References
-
- Bengtsson, K. L. et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine34, 1927–1935 (2016). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

