Impact of partial substitution of cisplatin with cyclophosphamide on acute toxicities in standard-risk medulloblastoma
- PMID: 40495013
- PMCID: PMC12263714
- DOI: 10.1007/s11060-025-05098-7
Impact of partial substitution of cisplatin with cyclophosphamide on acute toxicities in standard-risk medulloblastoma
Abstract
Purpose: Medulloblastoma (MB) treatment includes surgery, irradiation, and chemotherapy (CT). Cisplatin-based regimens for standard-risk (SR) MB are effective but associated with significant toxicities, particularly ototoxicity. This study compares the toxicity profiles of two CT regimens, focusing on grade ≥ 3 ototoxicity, hematologic, hepatic, renal, and neurologic toxicities.
Methods: This study included SR-MB patients aged 3-18 years. Cohort A (2016-2019) received adjuvant CT adopted from the Children's Oncology Group (COG) A9961 Regimen A protocol, with data collected retrospectively. Cohort B (2020-July 2022) received CT adopted from the ACNS0331 protocol, with data collected prospectively. Toxicities were assessed and graded using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
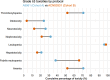
Results: A total of 168 patients aged 3 to 18 years were enrolled, 112 (67%) in cohort A and 56 (33%) in cohort B. Grade ≥ 3 ototoxicity was significantly higher in cohort A (24% vs. 3.6%, p < 0.001). Neurotoxicity occurred in 26% vs. 12.5% (p = 0.046). Anemia and thrombocytopenia in 71% vs. 52% (p = 0.04). Febrile neutropenia was more common in cohort B (66% vs. 38%, p < 0.001). No significant differences were found in grade ≥ 3 leukopenia, nephrotoxicity or hepatotoxicity. The 2-year overall survival was 96.4% (95% CI: 93.1-99.9) in cohort A vs. 86.6% (95% CI: 77.8-96.4) in cohort B (p = 0.11). Event-free survival was 92.9% (95% CI: 88.2-97.8) vs. 86.8% (95% CI: 78-96.4) (p = 0.29).
Conclusion: Partial substitution of cisplatin with cyclophosphamide showed a better toxicity profile, particularly for ototoxicity and neurotoxicity, with no significant difference in survival.
Keywords: Chemotherapy; Cisplatin; Cyclophosphamide; Medulloblastoma; Ototoxicity; Toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was approved by the Institutional Review Board (IRB) at CCHE (IRB number: 52/2020). No additional consent was required, as patients had already signed institutional chemotherapy-specific consent. Privacy and confidentiality of patients’ data were ensured. Competing interests: The authors declare no competing interests.
Figures
References
-
- Bailey S et al (2025) Medulloblastoma therapy: consensus treatment recommendations from SIOP-Europe and the European research network. EJC Paediatr Oncol 5:100205. 10.1016/j.ejcped.2024.100205 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous