Recurrent somatic mutation and progerin expression in early vascular aging of chronic kidney disease
- PMID: 40495018
- PMCID: PMC12176630
- DOI: 10.1038/s43587-025-00882-6
Recurrent somatic mutation and progerin expression in early vascular aging of chronic kidney disease
Abstract
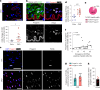
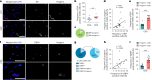
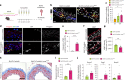
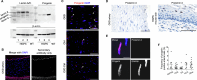
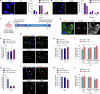
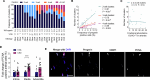
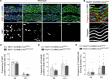
Early vascular aging plays a central role in chronic kidney disease (CKD), but its molecular causes remain unclear. Somatic mutations accumulate in various cells with age, yet their functional contribution to aging tissues is not well understood. Here we found progerin, the protein responsible for the premature aging disease Hutchinson-Gilford progeria syndrome, steadily recurring in vascular smooth muscle cells of patients with CKD. Notably, the most common progeria-causing mutation, LMNA c.1824C>T, was identified as a somatic mutation in CKD arteries. Clusters of proliferative progerin-expressing cells in CKD arteries and in vivo lineage-tracing in mice revealed clonal expansion capacity of mutant cells. Mosaic progerin expression contributed to genomic damage, endoplasmic reticulum stress and senescence in CKD arteries and resulted in vascular aging phenotypes in vivo. These findings suggest that certain somatic mutations may be clonally expanded in the arterial wall, contributing to the disease-related functional decline of the tissue.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
-
- Andreassi, M. G. Coronary atherosclerosis and somatic mutations: an overview of the contributive factors for oxidative DNA damage. Mutat. Res.543, 67–86 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

