PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy alone as the first line treatment for advanced biliary tract cancer: a pooled analysis of KEYNOTE-966 and TOPAZ-1 trails
- PMID: 40495210
- PMCID: PMC12150578
- DOI: 10.1186/s12957-025-03877-0
PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy alone as the first line treatment for advanced biliary tract cancer: a pooled analysis of KEYNOTE-966 and TOPAZ-1 trails
Abstract
Background: The efficacy of PD-1/PD-L1 inhibitors plus chemotherapy (PIC) as a first-line treatment for advanced biliary tract cancer (BTC) remains controversial, given inconsistent findings regarding survival benefits and safety concerns. This meta-analysis systematically evaluates the safety and efficacy of PIC versus chemotherapy alone, aiming to provide more definitive guidance for BTC treatment decisions.
Methods: To identify phase 3 randomized controlled trials (RCTs) comparing PIC with chemotherapy alone in patients with advanced BTC, a comprehensive literature search was conducted across six databases. Primary outcomes were overall survival (OS) and progression-free survival (PFS), while secondary outcomes included response rates and adverse events (AEs).
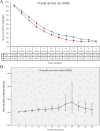
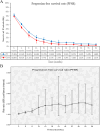
Results: Two phase 3 RCTs (KEYNOTE-966 and TOPAZ-1) involving 1,754 patients met the inclusion criteria. Findings demonstrated that the PIC group had significantly improved OS (hazard ratio [HR]: 0.76 [0.66, 0.87]) and PFS (HR: 0.76 [0.66, 0.87]). The mOS (MD: 1.70 [1.51, 1.90] months) and mPFS (MD: 1.20 [0.61, 1.79] months) were also higher in the PIC group. OS and PFS advantages for the PIC group were confirmed across most subgroups. In the first two years of treatment, survival rates (OS and PFS) and extended duration of response in the PIC group increased over time. Both groups showed comparable total AEs, treatment-related AEs, ORR, and DCR. However, the PIC group had significantly higher rates of immune-related AEs (irAEs) and grade 3-5 irAEs.
Conclusions: PIC is associated with improved OS and PFS in advanced BTC compared to chemotherapy alone, though the elevated risk of irAEs calls for careful monitoring.
Trial registration: PROSPERO ID: CRD42024611835. PROSPERO link: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024611835 .
Keywords: Biliary tract cancer; Chemotherapy; Meta-analysis; PD-1/PD-L1 inhibitors; Randomized controlled trials.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Due to the nature of this study no ethical approval was required. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- Scheithauer W. Chemotherapy: Gemcitabine alone or plus cisplatin for biliary tract cancer? Nat Rev Clin Oncol. 2010;7(10):554–5. - PubMed
-
- Rizzo A, Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert Rev Gastroenterol Hepatol. 2021;15(5):483–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

