Beyond the brain: early autonomic dysfunction in Alzheimer's disease
- PMID: 40495232
- PMCID: PMC12150586
- DOI: 10.1186/s40478-025-02042-8
Beyond the brain: early autonomic dysfunction in Alzheimer's disease
Abstract
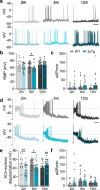
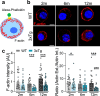
Alzheimer's disease (AD) is classically defined by central hallmarks such as amyloid-beta plaques, tau hyperphosphorylation, and synaptic failure. However, mounting evidence suggests that dysfunction outside the brain, particularly in the peripheral nervous system, may also play a significant role in disease progression. The adrenal medulla-a key regulator of systemic neurotransmission and stress response-has received little attention in this context. In this study, we investigated whether chromaffin cells (CCs) from the triple transgenic AD mouse model (3xTg) exhibit functional alterations that could contribute to peripheral neurochemical imbalance. Using electrophysiology, high-resolution amperometry, and neurotransmitter quantification, we identified early and progressive defects in CC function. Remarkably, even at two months of age-prior to cognitive decline-3xTg CCs showed impaired exocytosis, reduced vesicle release, and slower fusion pore kinetics. These changes were accompanied by diminished sodium (INa), calcium (ICa), and nicotinic (IACh) currents, compromising CC excitability. With age, a shift toward increased potassium (IK) currents and enhanced catecholamine secretion may reflect compensatory adaptations aimed at preserving output. These functional deficits were paralleled by structural remodeling of the actin cytoskeleton and systemic neurotransmitter disturbances. Noradrenaline levels increased in both plasma and brain, while dopamine decreased peripherally but paradoxically increased in the prefrontal cortex and hippocampus. Serotonin levels consistently declined across compartments. These imbalances correlated with altered behavior: 3xTg mice displayed increased exploration of exposed areas and heightened behavioral despair, pointing to anxiety- and depression-like phenotypes. Together, our findings identify the adrenal medulla as a previously underrecognized site of early catecholaminergic dysregulation in AD. The observed associations between peripheral CC dysfunction, systemic neurotransmitter imbalance, and behavioral changes point to a potential link between peripheral neuroendocrine alterations and central disease features. These results broaden the current understanding of AD pathophysiology and support the adrenal medulla as a promising candidate for further investigation as a therapeutic target and source of peripheral biomarkers.
Keywords: Alzheimer´s disease; Catecholamine release; Chromaffin cells; Electrophysiology; Neuropsychiatric symptoms; Peripheral dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Experiments were conducted according to the recommendation of the Ethics Committee from Universidad Autónoma de Madrid on the use of animals for laboratory experimentation, in accordance with the code of ethics and guidelines established by the European Community Directive (2010/63/EU) and Spanish legislation (RD53/2013). All efforts were made to avoid animal suffering and to use the minimum number of animals allowed by the experimental protocol and the statistical power of group data. Mice were housed under controlled temperature, a 12:12 h light cycle, and food and water were provided ad libitum. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. 10.1016/j.pneurobio.2013.06.005 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

