Engineering CRISPR System-Based Bacterial Outer Membrane Vesicle Potentiates T Cell Immunity for Enhanced Cancer Immunotherapy
- PMID: 40495695
- PMCID: PMC12506603
- DOI: 10.1002/adma.202501565
Engineering CRISPR System-Based Bacterial Outer Membrane Vesicle Potentiates T Cell Immunity for Enhanced Cancer Immunotherapy
Abstract
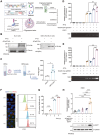
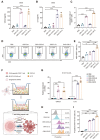
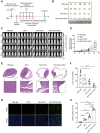
Immune checkpoint blockade (ICB) therapy has revolutionized cancer treatment but only benefits a subset of patients because of insufficient infiltration and inactivation of effector T cells. Bacterial outer membrane vesicles (OMVs) can activate immunity and deliver therapeutic agents for immunotherapy. However, efficiently targeting and packaging therapeutic molecules into OMVs remains challenging. Here, the engineered E. coli BL21-derived OMVs enable the packaging of multiple genes, resulting in a 7-fold increase in DNA enrichment efficiency and gene silencing in vitro. Moreover, the engineered OMVs carrying genes encoding CXCL9 and IL12 (OMV-C9I12) reprogram tumor cells to secrete these factors, significantly enhancing T-cell chemotaxis and activation. More importantly, this system markedly inhibits tumors, extends survival, and synergizes with anti-PD-1/PD-L1 therapy in murine MB49 and B16F10 tumor models. Single-cell RNA sequencing (scRNA-seq) further reveals significant upregulation of T-cell chemotaxis and activation-related pathways following OMV-C9I12 treatment. Finally, OMV-C9I12 potentiates T cell-mediated immunotherapy and suppresses the growth of bladder and breast cancer tumors in humanized mouse models. These findings highlight the potential of this engineered OMV platform for cancer gene therapy and provide novel strategies to overcome resistance to immunotherapy.
Keywords: CRISPR system; T cell immunity; cancer immunotherapy; gene therapy; outer membrane vesicles; self assembly.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) O'Donnell J. S., Teng M. W. L., Smyth M. J., Nat. Rev. Clin. Oncol. 2019, 16, 151; - PubMed
- b) Jansen C. S., Prokhnevska N., Master V. A., Sanda M. G., Carlisle J. W., Bilen M. A., Cardenas M., Wilkinson S., Lake R., Sowalsky A. G., Valanparambil R. M., Hudson W. H., McGuire D., Melnick K., Khan A. I., Kim K., Chang Y. M., Kim A., Filson C. P., Alemozaffar M., Osunkoya A. O., Mullane P., Ellis C., Akondy R., Im S. J., Kamphorst A. O., Reyes A., Liu Y., Kissick H., Nature 2019, 576, 465. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2018YFA0902800/National Key Research and Development Program of China
- 82322056/National Natural Science Foundation of China
- 82341018/National Natural Science Foundation of China
- 82072827/National Natural Science Foundation of China
- 82273421/National Natural Science Foundation of China
- 82303405/National Natural Science Foundation of China
- 82472137/National Natural Science Foundation of China
- 2023A03J0718/Science and Technology Program of Guangzhou
- 2024B03J1234/Science and Technology Program of Guangzhou
- 2024A04J6558/Science and Technology Program of Guangzhou
- Fundamental Research Funds for the Central Universities
- 23ykbj002/Sun Yat-sen University
- 2020B1111170006/Guangdong Provincial Clinical Research Centre for Urological Diseases
- 2020B1212060018/Guangdong Science and Technology Department
- 2018B030317001/Guangdong Science and Technology Department
- 2017B030314026/Guangdong Science and Technology Department
LinkOut - more resources
Full Text Sources
Medical
Research Materials

