Technical success, clinical efficacy, and safety of endoscopic minor papilla interventions for symptomatic pancreatic diseases
- PMID: 40495942
- PMCID: PMC12146942
- DOI: 10.3748/wjg.v31.i20.100192
Technical success, clinical efficacy, and safety of endoscopic minor papilla interventions for symptomatic pancreatic diseases
Abstract
Background: Endoscopic minor papilla intervention (EMPI) is an option for diagnosing or treating symptomatic pancreatic diseases in cases with failed pancreatic duct deep cannulation via the major papilla, pancreas divisum with obstruction of the minor papilla, or an abnormal patulous orifice of the minor papilla during endoscopic retrograde cholangiopancreatography (ERCP). However, the relatively low patency and small opening of the minor papillae pose technical challenges.
Aim: To evaluate the technical success, clinical success, stone clearance, and safety profile of EMPI for diagnosis and treatment of symptomatic pancreatic diseases.
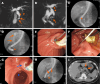
Methods: Patients diagnosed with symptomatic pancreatic diseases and EMPI between February 1996 and February 2023 were included. The primary outcomes were the initial technical success, defined as successful deep cannulation via the minor papilla (DCMP; access of the guidewire to the upstream pancreatic duct via the minor papilla) alone, overall technical success, defined as successful DCMP alone and successful DCMP with additional needle-knife precut minor papillotomy (NKPMP), and immediate clinical success, defined as > 50% improvement in abdominal pain after therapeutic EMPI. Secondary outcomes included long-term clinical success at 1, 3, and 7 years, pancreatic stone clearance, and procedure-related early and late adverse events (AEs).
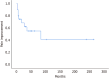
Results: Overall, 43 patients, 32 with obstructive chronic pancreatitis, 8 with pancreatic divisum, and 3 with intraductal papillary mucinous neoplasm were included. The initial and overall technical success rates were 74.4% (32/43) and 88.4% (38/43), respectively. The immediate clinical success rate was 79.1% (34/43), and the long-term clinical success rates at 1, 3, and 7 years were 74.7%, 55.3%, and 41.5%, respectively, among the 22 patients with a follow-up period of 57.5 (7-266) months. Complete and partial success of pancreatic stone clearance was achieved in 53.9% (7/13) and 15.4% (2/13), respectively. Early AEs included post-ERCP pancreatitis (PEP, n = 5) and self-limiting bleeding (n = 1); surgery therapy was required for 1 case with severe PEP and conservative management for the other 4 with mild PEP. Late AEs included minor papilla stricture (n = 1) after endoscopic minor papillotomy and pancreatic duct stricture (n = 1) after double pancreatic stent placement; no specific treatment was implemented for these events.
Conclusion: EMPI is feasible, effective, and safe for symptomatic pancreatic diseases, in terms of the technical and clinical success, stone clearance, and incidence and severity of AEs. NKPMP appears to enhance technical success. However, potential risks of developing PEP and late AEs should be kept in mind.
Keywords: Abdominal pain improvement; Endoscopic minor papilla intervention; Obstructive chronic pancreatitis; Pancreas division; Symptomatic pancreatic diseases.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures


References
-
- Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020;115:322–339. - PubMed
-
- Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J. 2017;5:153–199. - PMC - PubMed
-
- Singh A, Bush N, Bhullar FA, Faghih M, Moreau C, Mittal R, Seo JH, Talukdar R, Lakhtakia S, Singh VK, Akshintala VS. Pancreatic duct pressure: A review of technical aspects and clinical significance. Pancreatology. 2023;23:858–867. - PubMed
-
- Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179–193. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

