Decoding respiratory syncytial virus morphology: distinct structural and molecular signatures of spherical and filamentous particles
- PMID: 40496018
- PMCID: PMC12149210
- DOI: 10.3389/fcimb.2025.1597279
Decoding respiratory syncytial virus morphology: distinct structural and molecular signatures of spherical and filamentous particles
Abstract
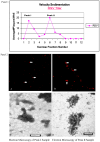
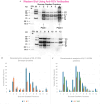
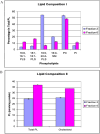
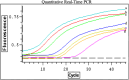
Respiratory syncytial virus (RSV) is a pleomorphic enveloped virus that buds as both spherical and filamentous particles. The determinants of RSV particle morphology and the roles of these forms in transmission and pathogenicity are not clearly defined, owing to a complex interplay of viral proteins and host factors that remains poorly understood. To further characterize RSV morphology, we developed a sucrose gradient velocity sedimentation method to separate spherical and filamentous virions. Fluorescence microscopy and electron microscopy (EM) confirmed two distinct peaks containing predominantly spherical or filamentous particles, respectively. Notably, EM images revealed a distinctive "honeycomb" pattern on the RSV envelope, suggesting an ordered lattice of glycoproteins on the virion surface. Biochemical analyses of viral protein and lipid content showed that filamentous particles contained higher levels of uncleaved fusion protein F0 and exhibited distinct phospholipid profiles compared to spherical particles. Both forms were enriched in cholesterol and phospholipids characteristic of lipid rafts, consistent with the idea that RSV buds from lipid raft microdomains. This enrichment in raft lipids is linked to cell-to-cell fusion (syncytium) formation and virion assembly. Quantitative real-time PCR analysis indicated a high particle-to-PFU ratio (~4:1), meaning only about one in four RSV virions was infectious. Spherical particles contained on average ~3 genomic RNA copies per virion, whereas filamentous particles contained ~2 copies. These data reveal several structural and compositional differences between RSV particle morphologies that may influence viral pathogenesis, and they provide a foundation for new antiviral approaches targeting virion assembly and morphology.
Keywords: filamentous particles; morphology; respiratory syncytial virus; spherical particles; sucrose gradient velocity sedimentation.
Copyright © 2025 Pastey, McCurdy and Graham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Folch J., Lees M., Sloane G. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. Available at: https://www.jbc.org/article/S0021-9258(18)64849-5/pdf (Accessed May 20, 2022; December 4, 2024; February 1, 2025). - PubMed
-
- Gower T. L., Pastey M. K., Peeples M. E., Collins P. L., McCurdy L. H., Hart T. K., et al. (2005). RhoA signaling is required for respiratory syncytial virus–induced syncytium formation and filamentous virion morphology. J. Virol. 79, 5326–5336. doi: 10.1128/JVI.79.9.5326-5336.2005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

