Perspectives on Molecular Mechanisms of Hydrate Formation and Growth at Interfaces: A Mini-Review
- PMID: 40496095
- PMCID: PMC12147157
- DOI: 10.1021/acs.energyfuels.5c00942
Perspectives on Molecular Mechanisms of Hydrate Formation and Growth at Interfaces: A Mini-Review
Abstract
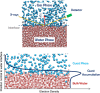
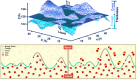
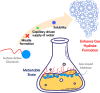
Hydrate-based engineering applications hold significant promise due to their physical feasibility and low energy consumption. However, key challengesincluding operating conditions, formation and growth rates, and gas storage capacitycontinue to impact their viability as sustainable technologies. This mini-review offers key insights into molecular mechanisms governing hydrate nucleation and growth at guest-water interfaces, specifically examining the role of mass transfer thermodynamics across the interface in either promoting or inhibiting gas hydrate formation. Additionally, this review highlights recent advancements, emerging research opportunities, and potential commercialization pathways for these technologies. With continued development, technologies utilizing hydrates have the capability to play a transformative role across multiple industries, offering a more sustainable alternative to existing commercial solutions.
© 2025 The Author. Published by American Chemical Society.
Figures

Similar articles
-
Advances in Nanomaterials for Sustainable Gas Separation and Storage: Focus on Clathrate Hydrates.Acc Chem Res. 2023 Nov 21;56(22):3111-3120. doi: 10.1021/acs.accounts.3c00406. Epub 2023 Nov 7. Acc Chem Res. 2023. PMID: 37934857
-
Temperature-Controlled Gas Hydrate Nucleation in the Heterogeneous Environment.J Phys Chem Lett. 2025 Jan 16;16(2):667-674. doi: 10.1021/acs.jpclett.4c03074. Epub 2025 Jan 9. J Phys Chem Lett. 2025. PMID: 39786985
-
Overview: Nucleation of clathrate hydrates.J Chem Phys. 2016 Dec 7;145(21):211705. doi: 10.1063/1.4968590. J Chem Phys. 2016. PMID: 28799342
-
Interfacial phenomena in gas hydrate systems.Chem Soc Rev. 2016 Mar 21;45(6):1678-90. doi: 10.1039/c5cs00791g. Chem Soc Rev. 2016. PMID: 26781172 Review.
-
"Nanoreactors" for Boosting Gas Hydrate Formation toward Energy Storage Applications.ACS Nano. 2022 Aug 23;16(8):11504-11515. doi: 10.1021/acsnano.2c04640. Epub 2022 Aug 8. ACS Nano. 2022. PMID: 35939085 Review.
References
-
- Dendy Sloan, E. ; Koh, C. A. . Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press - Taylor & Francis Group: 2007; p 752.
-
- Zerpa L. E., Salager J. L., Koh C. A., Sloan E. D., Sum A. K.. Surface Chemistry and Gas Hydrates in Flow Assurance. Ind. Eng. Chem. Res. 2011;50:188–197. doi: 10.1021/ie100873k. - DOI
-
- Striolo A., Phan A., Walsh M. R.. Molecular Properties of Interfaces Relevant for Clathrate Hydrate Agglomeration. Curr. Opin Chem. Eng. 2019;25:57–66. doi: 10.1016/j.coche.2019.08.006. - DOI
Publication types
LinkOut - more resources
Full Text Sources
