Re-assessment of the risks to public health related to the genotoxicity of styrene present in plastic food contact materials
- PMID: 40496253
- PMCID: PMC12149764
- DOI: 10.2903/j.efsa.2025.9473
Re-assessment of the risks to public health related to the genotoxicity of styrene present in plastic food contact materials
Abstract
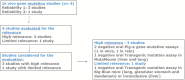
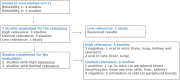
The EFSA Panel on Food Contact Materials (FCM) was requested by the European Commission to re-evaluate the potential genotoxicity of styrene after oral exposure and its safety for use in plastic FCM with a specific migration limit (SML) of 40 μg/kg food. A rigorous assessment of the in vivo genotoxicity studies (i) provided by third parties, (ii) identified by a targeted literature search and (iii) reported in the 2019 IARC Monograph was performed. All studies were assessed for reliability and relevance and the results integrated in the weight of evidence. The results provided by reliable in vivo oral genotoxicity studies, covering different genetic endpoints and target tissues, including liver, the primary site of metabolism, demonstrated that the oral administration of styrene in mice and rats up to the maximum tolerated dose (300 and 500 mg/kg body weight (bw), respectively) did not induce genotoxic effects. The Panel concluded that there was no evidence that styrene is genotoxic following oral exposure. For substances demonstrated to be non-genotoxic, according to the EFSA Note for Guidance for FCM, an SML up to 50 μg/kg food would not be of safety concern. Consequently, the use of styrene in the manufacture of FCM respecting the SML of 40 μg/kg food proposed by the European Commission is not of safety concern.
Keywords: CAS No 100‐42‐5; food contact materials; genotoxicity; oral exposure; safety assessment; styrene.
© 2025 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures

Similar articles
-
Assessment of the impact of the IARC Monograph Vol. 121 on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials.EFSA J. 2020 Oct 14;18(10):e06247. doi: 10.2903/j.efsa.2020.6247. eCollection 2020 Oct. EFSA J. 2020. PMID: 33133270 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Review and priority setting for substances that are listed without a specific migration limit in Table 1 of Annex 1 of Regulation 10/2011 on plastic materials and articles intended to come into contact with food.EFSA J. 2020 Jun 10;18(6):e06124. doi: 10.2903/j.efsa.2020.6124. eCollection 2020 Jun. EFSA J. 2020. PMID: 32874315 Free PMC article.
-
Safety assessment of the substance poly((R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate) for use in food contact materials.EFSA J. 2019 Jan 25;17(1):e05551. doi: 10.2903/j.efsa.2019.5551. eCollection 2019 Jan. EFSA J. 2019. PMID: 32626096 Free PMC article.
-
Genotoxicity testing approaches for the safety assessment of substances used in food contact materials prior to their authorization in the European Union.Environ Mol Mutagen. 2017 Jun;58(5):361-374. doi: 10.1002/em.22094. Epub 2017 May 28. Environ Mol Mutagen. 2017. PMID: 28556235 Review.
References
-
- Banton, M. I. , Bus, J. S. , Collins, J. J. , Delzell, E. , Gelbke, H. P. , Kester, J. E. , Moore, M. M. , Waites, R. , & Sarang, S. S. (2019). Evaluation of potential health effects associated with occupational and environmental exposure to styrene–an update [review]. Journal of Toxicology and Environmental Health ‐ Part B: Critical Reviews, 22(1–4), 1–130. 10.1080/10937404.2019.1633718 [RefID 1383]. - DOI - PubMed
-
- Beije, B. , & Jenssen, D. (1982). Investigation of styrene in the liver perfusion/cell culture system. No indication of styrene‐7,8‐oxide as the principal mutagenic metabolite produced by the intact rat liver. Chemico‐Biological Interactions, 39(1), 57–76. 10.1016/0009-2797(82)90006-0 - DOI - PubMed
-
- Bigbee, W. L. , Grant, S. G. , Langlois, R. G. , Jensen, R. H. , Anttila, A. , Pfäffli, P. , Pekari, K. , & Norppa, H. (1996). Glycophorin a somatic cell mutation frequencies in Finnish reinforced plastics workers exposed to styrene. Cancer Epidemiology, Biomarkers & Prevention, 5(10), 801–810. - PubMed
LinkOut - more resources
Full Text Sources
