PD-1 or PD-L1 inhibitors in addition to first-line chemotherapy for endometrial cancer: an extracted individual patient data meta-analysis
- PMID: 40496311
- PMCID: PMC12149239
- DOI: 10.3332/ecancer.2025.1884
PD-1 or PD-L1 inhibitors in addition to first-line chemotherapy for endometrial cancer: an extracted individual patient data meta-analysis
Abstract
Objective: To assess the impact of PD-1/PD-L1 inhibitors in first-line treatment of advanced or recurrent endometrial cancer (EC) through individual patient data (IPD) Meta-analysis, providing insights by integrated survival curves.
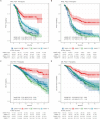
Methods: We searched PubMed, Embase, Cochrane and meetings up to April 2024 for randomised phase II or III trials (randomised controlled trials) investigating immunotherapy plus chemotherapy for EC. IPD was reconstructed from Kaplan-Meier plots using WebPlotDigitizer and the R package IPDfromKM, and then combined.
Results: NRG-GY018, RUBY, MITO END-3, AtTEnd/ENGOT-en7 and DUO-E were included. 2,436 patients were analysed for progression-free survival (PFS) and 2,317 for overall survival (OS). Among these, 621 patients had deficient DNA mismatch repair (dMMR) and 1,815 had the proficient disease (pMMR).The IPD analysis highlighted the significant benefit of adding immunotherapy to chemotherapy in dMMR patients, with 3-year absolute gains of 36% in PFS (HR 0.36, 95% CI 0.28-0.45) and 28% in OS (HR 0.41, 95% CI 0.30-0.48).For pMMR, a smaller benefit was observed in PFS, with a 3-year absolute gain of 6% (HR 0.78, 95% CI 0.69-0.88). Notably, a significant benefit occurred only with PD-1 inhibitors (PFS HR 0.66, 95% CI 0.55-0.79; OS: HR 0.78, 95% CI 0.62-0.96). No significant benefit was seen with PD-L1 inhibitors (PFS: 0.87, 95% CI 0.75-1.03; OS: HR: 0.93, 95% CI 0.75-1.16).
Conclusion: This meta-analysis validated the benefit of adding immunotherapy to platinum-based chemotherapy with respect to PFS. dMMR patients gain advantages from the inclusion of either anti-PD-1 or anti-PD-L1 agents, whereas pMMR patients only experience this benefit when treated with anti-PD-1 agents.
Keywords: advanced or recurrent endometrial cancer; extracted individual patient data; immunotherapy; meta-analysis.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
FLN, MTC and MMST: declare no competing interest. MCG: Speaker fees: Novartis, Knight Therapeutics; Financial support for educational programs and symposia: Pfizer, GSK. RCB: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Nestle Health Science, Zodiac, Gilead, MSD. Expert testimony: AstraZeneca, Ache, Nestle Health Science. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo. Institutional - Research grant: Novartis, AstraZeneca; MS: Speaker fees and/or honoraria for consulting or advisory functions: MSD, AstraZeneca, Libbs, GSK, Zodiac, Roche, Orentt, MDHealth, Sanofi, Gilead, Amgen, Sophia genetics.
Figures







References
-
- Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
