Strategies to accelerate cervical cancer elimination in Greece: a modeling study
- PMID: 40496621
- PMCID: PMC12148835
- DOI: 10.3389/fonc.2025.1480942
Strategies to accelerate cervical cancer elimination in Greece: a modeling study
Abstract
Introduction: Most cervical cancer cases are caused by human papillomavirus (HPV), a vaccine-preventable infection. According to the World Health Organization (WHO), both high HPV vaccination coverage and cervical cancer screening rates will accelerate the elimination of cervical cancer, a threshold defined as <4 age-standardized cases per 100,000 women.
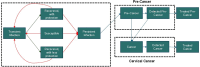
Methods: A dynamic transmission model was used to study the effect of increased HPV vaccination coverage and cervical cancer screening rates in Greece on cervical cancer incidence over a 100-year time horizon. Greek-specific or proxy data were used for both model inputs and calibration prior to the evaluation of eight different vaccination and screening scenarios. The estimated time to cervical cancer elimination and eradication in Greece was reported as the year each scenario reached <4 cases per 100,000 and <1 case per 100,000, respectively.
Results: Greece reached the WHO cervical cancer elimination threshold by 2074 with a 50% HPV vaccination coverage and 50% Pap test screening rate. When HPV DNA-based methods replaced Pap tests at the same rate and HPV vaccination coverage levels, the WHO threshold was reached by 2061. Other scenarios modeled future changes in HPV DNA-based screening rates with either 50% or 90% vaccination coverage. The 75% HPV DNA-based screening with 90% vaccination coverage scenario reached the WHO threshold by 2047 and the eradication threshold before the end of the century (2096).
Conclusion: If public health interventions are implemented to accelerate HPV vaccination coverage and HPV DNA-based screening adherence within the next five years, Greece can reach the WHO's cervical cancer elimination threshold by 2047 and eradicate cervical cancer before the end of the century.
Keywords: 9-valent; Greece; HPV; HPV DNA; cervical cancer; human papillomavirus vaccine; nonavalent; screening.
Copyright © 2025 Palmer, Skroumpelos, Sabale, Gountas, Trimis, Karokis and Agorastos.
Conflict of interest statement
CP is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may hold stock in Merck & Co., Inc., Rahway, NJ, USA. AS, IG, GT, and AK are employees of MSD Greece who may own stock and/or hold stock options in in Merck & Co., Inc., Rahway., NJ, USA. US is an employee of MSD Lithuania who may own stock and/or hold stock options in in Merck & Co., Inc., Rahway, NJ, USA. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder of the study had a role in the study design, collection, analysis, interpretation of the data, the writing of this article or the decision to submit it for publication.
Figures

References
-
- Cancer Research UK . Does HPV cause Cancer? 2021 (2021). Available online at: https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/infection... (Accessed December 1, 2023).
-
- World Health Organization . Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; (2020). Available at: https://www.who.int/publications/i/item/9789240014107 (Accessed March 30, 2023).
-
- Bruni L AG, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, et al. Human papillomavirus and related diseases in the world. Summary report. ICO/IARC Information Centre on Papillomavirus (HPV) and Cancer. Barcelona: Cancer Epidemiology Research Programme Institut Català d'Oncologia (ICO). Av. Gran Via de l'Hospitalet L'Hospitalet de Llobregat, Barcelona, 08908: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre; (2023). Available at: https://hpvcentre.net/datastatistics.php.
-
- World Health Organization . Cervical cancer (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed December 1, 2023).
LinkOut - more resources
Full Text Sources
Research Materials

