Quantum machine learning-based electrokinetic mining for the identification of nanoparticles and exosomes with minimal training data
- PMID: 40496630
- PMCID: PMC12149654
- DOI: 10.1016/j.bioactmat.2025.03.023
Quantum machine learning-based electrokinetic mining for the identification of nanoparticles and exosomes with minimal training data
Abstract
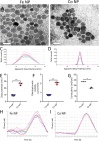
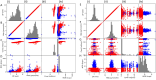
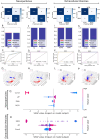
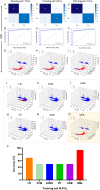
Synthetic and naturally occurring particles, such as nanoparticles (NPs) and exosomes; a type of extracellular vesicles (EVs), have garnered widespread attention across various fields, including biomaterials, oncology, and delivery systems for drugs and vaccines. Traditional methods for identifying NPs and EVs, such as transmission electron microscopy, are often prohibitively expensive and labor-intensive. As an alternative, the assessment of electrokinetic attributes such as zeta potential or electrophoretic mobility, conductance, and mean count rate, offers a more cost-effective, rapid, and reliable means of characterizing these particles. In this context, we introduce the first application of a quantum machine learning (QML)-based electrokinetic mining for the identification of green-synthesized iron- and cobalt-based NPs, as well as exosomes derived from human embryonic stem cells (hESC), human lung cancer (A549) cells, and colorectal cancer (CRC) cells, based solely on their electrokinetic attributes. Comparative analyses involving cross-validation, train-test splits, confusion matrices, and Receiver Operating Characteristic (ROC) curves revealed that classical ML techniques could accurately identify the types of NPs and EVs. Notably, QML demonstrated proficiency in differentiating between various NPs and EVs, including the distinction of EVs in the plasma of CRC patients versus those of healthy individuals. Furthermore, QML's application has been extended to the identification of NPs along with EVs in the plasma of CRC patients and experimental mice, achieving higher prediction performance even with a minimal training dataset, demonstrating that QML based electrokinetic mining could identify NPs or EVs with minimal training data, thereby facilitating novel clinical development in the realm of liquid biopsies.
Keywords: Electrokinetic analysis; Extracellular vesicle; Liquid biopsy; Machine learning; Nanoparticle; Quantum machine learning.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
[Efficient capture and proteomics analysis of urinary extracellular vesicles by affinity purification].Se Pu. 2025 May;43(5):508-517. doi: 10.3724/SP.J.1123.2024.11013. Se Pu. 2025. PMID: 40331614 Free PMC article. Chinese.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Human Plasma Extracellular Vesicle Isolation and Proteomic Characterization for the Optimization of Liquid Biopsy in Multiple Myeloma.Methods Mol Biol. 2021;2261:151-191. doi: 10.1007/978-1-0716-1186-9_10. Methods Mol Biol. 2021. PMID: 33420989
-
Molecular profiling of blood plasma-derived extracellular vesicles derived from Duchenne muscular dystrophy patients through integration of FTIR spectroscopy and machine learning reveals disease signatures.Spectrochim Acta A Mol Biomol Spectrosc. 2025 Feb 5;326:125236. doi: 10.1016/j.saa.2024.125236. Epub 2024 Oct 1. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 39368178
-
Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles.Acta Biomater. 2021 Oct 15;134:13-31. doi: 10.1016/j.actbio.2021.07.027. Epub 2021 Jul 18. Acta Biomater. 2021. PMID: 34284151 Review.
References
-
- Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M. del P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. - DOI - PMC - PubMed
-
- Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2017 doi: 10.1016/j.arabjc.2017.05.011. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

