Longitudinal neuromelanin changes in prodromal and early Parkinson's disease in humans and rat model
- PMID: 40496671
- PMCID: PMC12149847
- DOI: 10.1093/braincomms/fcaf204
Longitudinal neuromelanin changes in prodromal and early Parkinson's disease in humans and rat model
Abstract
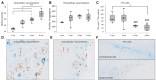
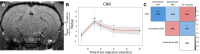
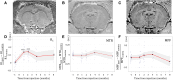
Neuromelanin-sensitive MRI has been proposed as a biomarker of Parkinson's disease pathology. However, the biological and physical origins of this contrast are debated. A recent rodent model of controlled neuromelanin accumulation in the substantia nigra has been developed and recapitulates several features of Parkinson's disease. In this work, we first combined neuromelanin-sensitive-MRI and histology to study neuromelanin accumulation and neurodegeneration in a humanized rat model of Parkinson's disease. Neuromelanin-sensitive-MRI signal changes were biphasic with an initial increase due to the accumulation of neuromelanin in dopaminergic neurons, followed signal decrease due to neurodegeneration. In healthy subjects and patients with isolated rapid eye movement sleep behaviour disorder, neuromelanin-sensitive-MRI signal increased initially and then decreased similarly as in rodents after reaching a similar maximum signal intensity in both groups. In early Parkinson's disease and converted isolated rapid eye movement sleep behaviour disorder patients, neuromelanin-sensitive-MRI signal drop was greater than in healthy individuals. Results in animals and humans show that neuromelanin-sensitive-MRI is a marker of the intracellular neuromelanin accumulation and then of neuronal degeneration and originates mainly from T1 reduction effect of neuromelanin.
Keywords: Parkinson’s disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.-C.C. has served in advisory boards for Alzprotect, Bayer, Biogen, Denali, Ferrer, Idorsia, iRegene, Prevail Therapeutic, Roche, Servier, Theranexus, UCB and received grants from Sanofi and the Michael J. Fox Foundation outside of this work. The other authors have no conflict of interests to declare.
Figures




References
-
- Braak H, Tredici KD, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. - PubMed
-
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334(6180):345–348. - PubMed
-
- Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG. A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology. 2006;67(7 Suppl 2):S8–S11. - PubMed
-
- Beach TG, Sue LI, Walker DG, et al. Marked microglial reaction in normal aging human substantia nigra: Correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114(4):419–424. - PubMed
LinkOut - more resources
Full Text Sources
