Magnetic Resonance-Guided Focused Ultrasound Neurosurgery for Treatment-Refractory Obsessive-Compulsive Disorder: A Health Technology Assessment
- PMID: 40496675
- PMCID: PMC12148002
Magnetic Resonance-Guided Focused Ultrasound Neurosurgery for Treatment-Refractory Obsessive-Compulsive Disorder: A Health Technology Assessment
Abstract
Background: Obsessive-compulsive disorder (OCD) is a debilitating neuropsychiatric illness characterized by obsessions and compulsions that are distressing, impair function, and are time-consuming, especially in severe cases. Up to 40% of people with OCD have treatment-refractory OCD and experience inadequate response to multiple trials and combinations of treatments. Neurosurgery is an important treatment option for people with severe, treatment-refractory OCD but is typically invasive. Magnetic resonance-guided focused ultrasound (MRgFUS) is a noninvasive technology that is used to perform neurosurgery. We conducted a health technology assessment of MRgFUS neurosurgery for people with severe, treatment-refractory OCD, which included an evaluation of effectiveness, safety, the budget impact of publicly funding MRgFUS neurosurgery, and patient preferences and values.
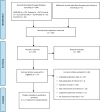
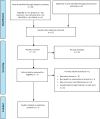
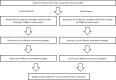
Methods: We performed a systematic literature search of the clinical evidence published since 2013. We assessed the risk of bias of each included study using the Joanna Briggs Institute's Critical Appraisal Checklist for Case Series, and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic literature search of the economic evidence. We estimated the 5-year budget impact of publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario. Owing to a lack of comparative clinical evidence, we did not conduct a primary economic evaluation. To contextualize the value of MRgFUS neurosurgery, we spoke to people with treatment-refractory OCD who underwent the procedure, as well as those on the waitlist.
Results: We included 2 studies in the clinical evidence review. In these small case series, MRgFUS neurosurgery led to improvements in OCD symptoms, quality of life, and patient functioning, as well as treatment response for many but not all patients (GRADE: Very low). In a minority of cases, the procedure could not be successfully performed due to skull factors (GRADE: Very low). MRgFUS neurosurgery was also found to have a favourable safety profile (GRADE: Very low). No cases of re-treatment were reported (GRADE: Very low). No studies compared MRgFUS neurosurgery with other neurosurgeries.Due to the lack of comparative clinical evidence, the cost-effectiveness of MRgFUS neurosurgery could not be determined. Our budget impact analysis found that publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would cost an additional $1.9 million over 5 years.Patients reported the negative impacts that OCD had on their day-to-day activities, work and school, social life and family relationships, and mental health. The 6 participants who underwent MRgFUS neurosurgery commented on the positive impact that it had on their OCD symptoms, mental health, and quality of life.
Conclusions: MRgFUS neurosurgery may be an effective and generally safe treatment option for severe, treatment-refractory OCD, but the evidence is very uncertain. The cost-effectiveness of MRgFUS neurosurgery could not be determined given the lack of comparative clinical evidence. Publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would result in an additional cost of $1.9 million over 5 years. Patients and care partners emphasized the negative impact of OCD in their lives and highlighted the importance of having access to MRgFUS neurosurgery as a treatment option for treatment-refractory OCD.
Copyright © King's Printer for Ontario, 2025.
Figures
Similar articles
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
-
Supplemental Screening as an Adjunct to Mammography for Breast Cancer Screening in People With Dense Breasts: A Health Technology Assessment.Ont Health Technol Assess Ser. 2023 Dec 19;23(9):1-293. eCollection 2023. Ont Health Technol Assess Ser. 2023. PMID: 39364436 Free PMC article.
-
Level 2 Polysomnography for the Diagnosis of Sleep Disorders: A Health Technology Assessment.Ont Health Technol Assess Ser. 2024 Aug 20;24(7):1-157. eCollection 2024. Ont Health Technol Assess Ser. 2024. PMID: 39372311 Free PMC article.
-
Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Valve Stenosis at Low Surgical Risk: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Nov 2;20(14):1-148. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 33240455 Free PMC article.
-
Internet-Delivered Cognitive Behavioural Therapy for Major Depression and Anxiety Disorders: A Health Technology Assessment.Ont Health Technol Assess Ser. 2019 Feb 19;19(6):1-199. eCollection 2019. Ont Health Technol Assess Ser. 2019. PMID: 30873251 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: The Association; 2013.
-
- DynaMed. Obsessive–compulsive disorder (OCD) [Internet]. EBSCO Information Services; c2023. [cited 2023 Jul 5]. Available from: https://www.dynamed.com/condition/obsessive–compulsive-disorder-ocd
-
- Hirschtritt ME, Bloch MH, Mathews CA. Obsessive–compulsive disorder: advances in diagnosis and treatment. JAMA. 2017; 317(13):1358–67. - PubMed
-
- Kuhne F, Ay DS, Marschner L, Weck F. The heterogeneous course of OCD - a scoping review on the variety of definitions. Psychiatry Res. 2020; 285:112821. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical