Differential Ca2+ handling by isolated synaptic and non-synaptic mitochondria: roles of Ca2+ buffering and efflux
- PMID: 40496698
- PMCID: PMC12149423
- DOI: 10.3389/fnsyn.2025.1562065
Differential Ca2+ handling by isolated synaptic and non-synaptic mitochondria: roles of Ca2+ buffering and efflux
Abstract
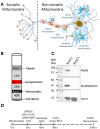
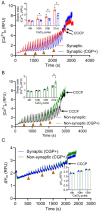
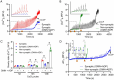
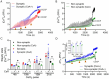
Mitochondria regulate intracellular calcium ion (Ca2+) signaling by a fine-tuned process of mitochondrial matrix (m) Ca2+ influx, mCa2+ buffering (sequestration) and mCa2+ release (Ca2+ efflux). This process is critically important in the neurosynaptic terminal, where there is a simultaneous high demand for ATP utilization, cytosolic (c) Ca2+ regulation, and maintenance of ionic gradients across the cell membrane. Brain synaptic and non-synaptic mitochondria display marked differences in Ca2+ retention capacity. We hypothesized that mitochondrial Ca2+ handling in these two mitochondrial populations is determined by the net effects of Ca2+ uptake, buffering or efflux with increasing CaCl2 boluses. We found first that synaptic mitochondria have a more coupled respiration than non-synaptic mitochondria; this may correlate with the higher local energy demand in synapses to support neurotransmission. When both mitochondrial fractions were exposed to increasing mCa2+ loads we observed decreased mCa2+ sequestration in synaptic mitochondria as assessed by a significant increase in the steady-state free extra matrix Ca2+ (ss[Ca2+]e) compared to non-synaptic mitochondria. Since, non-synaptic mitochondria displayed a significantly reduced ss[Ca2+]e, this suggested a larger mCa2+ buffering capacity to maintain [Ca2+]m with increasing mCa2+ loads. There were no differences in the magnitude of the transient depolarizations and repolarizations of the membrane potential (ΔΨm) and both fractions exhibited similar gradual depolarization of the baseline ΔΨm during additional CaCl2 boluses. Adding the mitochondrial Na+/Ca2+ exchanger (mNCE) inhibitor CGP37157 to the mitochondrial suspensions unmasked the mCa2+ sequestration and concomitantly lowered ss[Ca2+]e in synaptic vs. non-synaptic mitochondria. Adding complex V inhibitor oligomycin plus ADP (OMN + ADP) bolstered the matrix Ca2+ buffering capacity in synaptic mitochondria, as did Cyclosporin A (CsA), in non-synaptic. Our results display distinct differences in regulation of the free [Ca2+]m to prevent collapse of ΔΨm during mCa2+ overload in the two populations of mitochondria. Synaptic mitochondria appear to rely mainly on mCa2+ efflux via mNCE, while non-synaptic mitochondria rely mainly on Pi-dependent mCa2+ sequestration. The functional implications of differential mCa2+ handling at neuronal synapses may be adaptations to cope with the higher metabolic activity and larger mCa2+ transients at synaptosomes, reflecting a distinct role they play in brain function.
Keywords: Ca2+ buffering; Ca2+ efflux; bioenergetics; non-synaptic mitochondria; synaptic mitochondria.
Copyright © 2025 Mishra, Bevers, Li, Zare, Heisner, Tong, Kwok, Stowe and Camara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Slow Ca2+ Efflux by Ca2+/H+ Exchange in Cardiac Mitochondria Is Modulated by Ca2+ Re-uptake via MCU, Extra-Mitochondrial pH, and H+ Pumping by FOF1-ATPase.Front Physiol. 2019 Feb 4;9:1914. doi: 10.3389/fphys.2018.01914. eCollection 2018. Front Physiol. 2019. PMID: 30804812 Free PMC article.
-
Total Matrix Ca2+ Modulates Ca2+ Efflux via the Ca2+/H+ Exchanger in Cardiac Mitochondria.Front Physiol. 2020 Sep 16;11:510600. doi: 10.3389/fphys.2020.510600. eCollection 2020. Front Physiol. 2020. PMID: 33041851 Free PMC article.
-
Cyclosporin A Increases Mitochondrial Buffering of Calcium: An Additional Mechanism in Delaying Mitochondrial Permeability Transition Pore Opening.Cells. 2019 Sep 7;8(9):1052. doi: 10.3390/cells8091052. Cells. 2019. PMID: 31500337 Free PMC article.
-
Mitochondrial calcium and the regulation of metabolism in the heart.J Mol Cell Cardiol. 2015 Jan;78:35-45. doi: 10.1016/j.yjmcc.2014.10.019. Epub 2014 Nov 7. J Mol Cell Cardiol. 2015. PMID: 25450609 Free PMC article. Review.
-
Mitochondrial calcium at the synapse.Mitochondrion. 2021 Jul;59:135-153. doi: 10.1016/j.mito.2021.04.006. Epub 2021 Apr 23. Mitochondrion. 2021. PMID: 33895346 Review.
References
-
- Aldakkak M., Camara A. K., Heisner J. S., Yang M., Stowe D. F. (2011). Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol. Res. 64, 381–392. doi: 10.1016/j.phrs.2011.06.018, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

