A 'two-missile' nanoplatform for targeting triple-negative breast cancer: prodrug activation and immune enhancement
- PMID: 40496718
- PMCID: PMC12150181
- DOI: 10.1016/j.mtbio.2025.101891
A 'two-missile' nanoplatform for targeting triple-negative breast cancer: prodrug activation and immune enhancement
Abstract
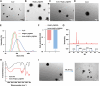
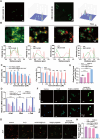
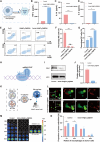
Targeted cancer therapy remains a significant challenge due to the complexity of tumor microenvironments and the need for precise drug delivery systems that can overcome these obstacles. To address this challenge, a nanoparticle platform based on prodrug activation and immune enhancement for "two-missile" targeting of TNBC has been developed: Azido-HA@Cu2O@DNA. This nanoparticle platform utilises azido hyaluronic acid (Azido-HA) as the primary carrier to enhance specificity to tumor tissues and targeting to tumor cells. The bioorthogonal reaction between the azido moiety and dibenzocyclooctyne-doxorubicin (DBCO-DOX) has been employed to improve the tumor targeting efficiency of chemotherapy is significantly enhanced, while the addition of copper ions promotes a Fenton-like reaction that induces cellular copper death. This mechanism not only spawns reactive oxygen species (ROS) for potent chemokinetic therapy but also facilitates the transition of macrophages from the M2 to the M1 phenotype, thereby bolstering anti-tumor immune responses. In addition, the platform's accompanying CRISPR/Cas9 system can reprogram tumor cells by down-regulating CD47 expression, enhancing macrophage recognition and phagocytosis activity, which in turn amplifies the efficacy of ensuing immunotherapy. The Azido-HA@Cu2O@DNA nanoplatform demonstrates excellent biocompatibility and safety, offering a promising strategy for enhancing tumor treatment. This methodology integrates the precise activation of prodrugs, immune system modulation, and macrophage-mediated tumor eradication, offering a holistic and potent paradigm for cancer therapy.
Keywords: Bioorthogonal reaction; CD47; CRISPR/Cas9; Cuproptosis; Macrophage M1 polarization; Macrophage phagocytosis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
A Gene-Editable Palladium-Based Bioorthogonal Nanoplatform Facilitates Macrophage Phagocytosis for Tumor Therapy.Angew Chem Int Ed Engl. 2023 Dec 11;62(50):e202313968. doi: 10.1002/anie.202313968. Epub 2023 Nov 10. Angew Chem Int Ed Engl. 2023. PMID: 37884479
-
Copper-Based Composites Nanoparticles Improve Triple-Negative Breast Cancer Treatment with Induction of Apoptosis-Cuproptosis and Immune Activation.Adv Healthc Mater. 2024 Nov;13(28):e2401646. doi: 10.1002/adhm.202401646. Epub 2024 Jul 12. Adv Healthc Mater. 2024. PMID: 39001628
-
Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer.J Immunother Cancer. 2021 Mar;9(3):e002022. doi: 10.1136/jitc-2020-002022. J Immunother Cancer. 2021. PMID: 33753567 Free PMC article.
-
A polymeric nanoplatform enhances the cGAS-STING pathway in macrophages to potentiate phagocytosis for cancer immunotherapy.J Control Release. 2024 Sep;373:447-462. doi: 10.1016/j.jconrel.2024.07.039. Epub 2024 Jul 25. J Control Release. 2024. PMID: 39038546
-
Dual-Prodrug-Based Hyaluronic Acid Nanoplatform Provides Cascade-Boosted Drug Delivery for Oxidative Stress-Enhanced Chemotherapy.ACS Appl Mater Interfaces. 2024 Sep 25;16(38):50459-50473. doi: 10.1021/acsami.4c11662. Epub 2024 Sep 11. ACS Appl Mater Interfaces. 2024. PMID: 39258403
References
LinkOut - more resources
Full Text Sources
Research Materials

