A triple-targeting "nano-brake" remodeling the impaired immune microenvironment in skin lesions for psoriasis treatment
- PMID: 40496725
- PMCID: PMC12148668
- DOI: 10.1016/j.mtbio.2025.101875
A triple-targeting "nano-brake" remodeling the impaired immune microenvironment in skin lesions for psoriasis treatment
Abstract
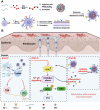
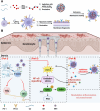
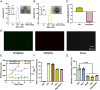
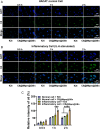
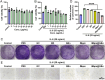
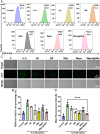
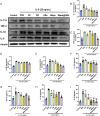
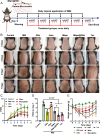
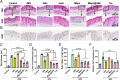
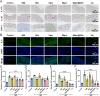
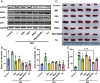
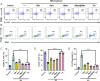
Psoriasis, a prevalent immune-mediated chronic inflammatory skin ailment, has been linked to heightened oxidative stress and compromised immune tolerance. Immune checkpoint pathways, particularly the programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) signaling axis, are instrumental in establishing and sustaining self-tolerance and regulating immune responses. Augmenting PD-1/PD-L1 interaction holds promise for curtailing the proliferation and activation of infiltrating T cells and curbing the release of inflammatory cytokines, thereby mitigating psoriasis induced by impaired immune tolerance. Consequently, neutralizing the surplus reactive oxygen species (ROS) in the affected skin and revitalizing local immune tolerance could represent a beneficial approach to psoriasis treatment. In light of these insights, this study introduced a bilirubin-based nanoparticle cloaked in IFN-γ-stimulated macrophage membrane (designated as IMφm@GBn or "nano-brake"). Treatment with IFN-γ conferred the macrophage membrane with heightened expression of pro-inflammatory cytokine receptor and PD-L1. As a result, the engineered IMφm@GBn not only scavenged excessive ROS in psoriatic lesions but crucially also absorbed a wide spectrum of pro-inflammatory cytokines. Furthermore, it inhibited the proliferation and activation of infiltrating T cells through augmented PD-1/PD-L1 interactions, thereby rebalancing the Th17/Treg ratio. In an in vivo psoriasis mouse model, the "nano-brake" was locally and accurately delivered to the dermis via microneedle, orchestrating the immune microenvironment of psoriasis and mitigating autoimmune damage linked to impaired immune tolerance. This approach significantly enhanced the therapeutic efficacy in ameliorating psoriasis-associated symptoms. This study sets an illuminating precedent for cell membrane-based biomimetic nano-formulations, holding broad implications for psoriasis treatment through multi-pronged immunomodulation.
Keywords: Bilirubin; PD-1/PDL-1; Psoriasis; ROS; Th17/Treg.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Tumor-Derived PD-L1+ Exosomes with Natural Inflammation Tropism for Psoriasis-Targeted Treatment.Bioconjug Chem. 2023 Apr 10. doi: 10.1021/acs.bioconjchem.3c00129. Online ahead of print. Bioconjug Chem. 2023. PMID: 37036892
-
Effect of baricitinib in regulating programmed death 1 and ligand programmed cell death ligand 1 through JAK/STAT pathway in psoriasis.Indian J Pharmacol. 2022 May-Jun;54(3):183-193. doi: 10.4103/ijp.ijp_1089_20. Indian J Pharmacol. 2022. PMID: 35848689 Free PMC article.
-
Drug-loaded microneedle patches containing regulatory T cell-derived exosomes for psoriasis treatment.Acta Biomater. 2025 May 15;198:452-466. doi: 10.1016/j.actbio.2025.04.015. Epub 2025 Apr 8. Acta Biomater. 2025. PMID: 40210183
-
A Systematic Review on the Therapeutic Potentiality of PD-L1-Inhibiting MicroRNAs for Triple-Negative Breast Cancer: Toward Single-Cell Sequencing-Guided Biomimetic Delivery.Genes (Basel). 2021 Aug 4;12(8):1206. doi: 10.3390/genes12081206. Genes (Basel). 2021. PMID: 34440380 Free PMC article.
-
Progress of research on PD-1/PD-L1 in leukemia.Front Immunol. 2023 Sep 26;14:1265299. doi: 10.3389/fimmu.2023.1265299. eCollection 2023. Front Immunol. 2023. PMID: 37822924 Free PMC article. Review.
Cited by
-
Systemic Psoriasis: From Molecular Mechanisms to Global Management Strategies.Clin Rev Allergy Immunol. 2025 Aug 7;68(1):79. doi: 10.1007/s12016-025-09089-4. Clin Rev Allergy Immunol. 2025. PMID: 40775488 Review.
References
-
- Nellikode D.A.S. Effiffifficacy of shodhana and sarvang takradhara therapy in the management of psoriasis A case study. International Journal of Preclinical & Clinical Research. 2023;4(1):15–20.
-
- Xu Q., Du J., Cao W., Sun S. Demographic and epidemiological drivers of global burden of psoriasis. Australas. J. Dermatol. 2021;62:e554–e558. - PubMed
-
- Le A.M., Torres T. New topical therapies for psoriasis. Am. J. Clin. Dermatol. 2022;23:13–24. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

