Recent advances in NIR-II photothermal and photodynamic therapies for drug-resistant wound infections
- PMID: 40496729
- PMCID: PMC12148679
- DOI: 10.1016/j.mtbio.2025.101871
Recent advances in NIR-II photothermal and photodynamic therapies for drug-resistant wound infections
Abstract
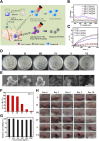
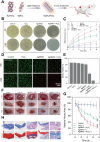
Bacterial infection can delay wound healing, while drug resistance further complicates the treatment of wound infection. Phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT), is a non/mini-invasive and efficient antibacterial strategy that rarely induces bacterial resistance. This treatment relies on specific wavelengths of light to activate photothermal agents (PTAs) or photosensitizers, killing bacteria by generating local heats or reactive oxygen species (ROS), respectively. However, the light for traditional PTT/PDT mainly falls in the visible and near-infrared I light (Vis/NIR-I light, 400-900 nm) regions, which significantly limits further clinical translations due to its low tissue permeability. The near-infrared II (NIR-II,1000-1700 nm) light is increasingly utilized in antibacterial PTT/PDT to improve tissue penetration and ameliorate the immune microenvironment of deeper wounds. Meanwhile, NIR-II light offers a higher maximum permissible exposure (MPE) for PTT/PDT in treating wound infections, thereby facilitating the security, in comparison to Vis/NIR-I light. This review highlights recent advancements in NIR-II PTT/PDT for drug-resistant wound infections, focusing on mechanisms, therapeutic outcomes, challenges, and prospects.
Keywords: Near-infrared II; Photodynamic therapy; Photothermal therapy; Wound healing; Wound infections.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











References
LinkOut - more resources
Full Text Sources
Miscellaneous

