Plasmon-activated water enhances gut-barrier function and alleviates inflammation in a mouse model of ulcerative colitis
- PMID: 40496763
- PMCID: PMC12150364
- DOI: 10.3892/etm.2025.12896
Plasmon-activated water enhances gut-barrier function and alleviates inflammation in a mouse model of ulcerative colitis
Abstract
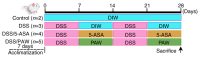
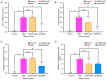
Ulcerative colitis (UC) is a chronic, relapsing inflammatory bowel disease characterized by disruption of the intestinal epithelial barrier and alterations in mucosal gene expression associated with intestinal integrity. Given the risks associated with UC, novel therapies capable of restoring intestinal barrier function and inhibiting inflammation are needed. Plasmon-activated water (PAW) is a nontoxic form of water with potential in the treatment of inflammatory diseases. The aim of the present study was to evaluate the therapeutic effects of PAW in a murine model of UC. Histological and immunohistochemical analyses were performed on colon tissues from mice with dextran sodium sulfate (DSS)-induced UC treated with either 5-aminosalicylic acid (5-ASA) or PAW. Epithelial cell density was decreased in the DSS model mice compared with that in the normal control mice, whereas treatment with 5-ASA or PAW attenuated this DSS-induced reduction. Microscopy revealed that the DSS/PAW group exhibited significantly reduced epithelial loss, crypt damage and inflammatory cell infiltration compared with that in the DSS group. In addition, immunohistochemical analysis demonstrated that PAW downregulated the DSS-induced expression of tumor necrosis factor-α and keratin 20 in epithelial cells and the lamina propria. Furthermore, PAW also attenuated the DSS-induced loss of expression of three proteins essential for cell adhesion and tight junctions, namely E-cadherin (CDH1), tight junction protein 1 (ZO-1) and occludin, in the colonic epithelium, particularly in intestinal crypts. In addition, mucin 1 (MUC1) expression was decreased and MUC2 expression increased in the mucosal layer of the colons of the DSS/PAW group compared with those in the DSS group. In conclusion, the colonic mucosa is a reliable site for evaluating epithelial damage and inflammatory infiltration. PAW ameliorated DSS-induced UC in mice by modulating the expression of key barrier-associated proteins, including CDH1, occludin, ZO-1, MUC1 and MUC2. These findings highlight the therapeutic potential of PAW in the treatment of colitis.
Keywords: 5-ASA; PAW; UC; gut barrier; inflammation.
Copyright: © 2025 Chang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
