Cannabinoid overrides triggers of GABAergic plasticity in vestibular circuits and distorts the development of navigation
- PMID: 40496815
- PMCID: PMC12150048
- DOI: 10.1016/j.isci.2025.112566
Cannabinoid overrides triggers of GABAergic plasticity in vestibular circuits and distorts the development of navigation
Abstract
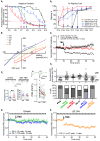
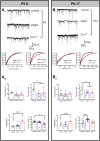
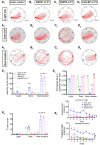
Early life exposure to cannabis can result in long-lasting deficits in spatial navigation. We ask if the development of this behavior is subject to early life activity of type I cannabinoid receptor (CB1R) in the vestibular nucleus. In rodents, we found that local exposure to CB1R agonist within the first postnatal week, but not thereafter, led to a decline in the induction efficacy of long-term depression at GABAergic synapses (LTDGABA), a key step in the hard-wiring of vestibular circuits. Within this critical period, endocannabinoid-mediated LTDGABA at inhibitory neurons was selectively triggered by cholecystokinin, whereas that at excitatory neurons was by serotonin. Neonatal exposure to cannabinoids extended the phase of high GABAergic synaptic plasticity and overrode the synapse-specific, modulatory mechanism for plasticity. Such treatment delayed the postnatal emergence of vestibular-dependent reflexes and deranged adult navigational behavior. Deficits in higher functions are thus attributable to the maldevelopment of sensory processing circuits resulting from early cannabis exposure.
Keywords: Biological sciences; Natural sciences; Neuroscience.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Lai S.K., Lai S.-K., Wu K.L.K., Ma C.-W., Ng K.-P., Hu X.-Q., Tam K.-W., Yung W.-H., Wang Y.T., Wong T.P., Shum D.K.-Y., et al. Timely insertion of AMPA receptors in developing vestibular circuits is required for manifestation of righting reflexes and effective navigation. Prog. Neurobiol. 2023;221:102402. doi: 10.1016/j.pneurobio.2023.102402. - DOI - PubMed
LinkOut - more resources
Full Text Sources

