CHD8 Variant and Rett Syndrome: Overlapping Phenotypes, Molecular Convergence, and Expanding the Genetic Spectrum
- PMID: 40496977
- PMCID: PMC12149508
- DOI: 10.1155/humu/5485987
CHD8 Variant and Rett Syndrome: Overlapping Phenotypes, Molecular Convergence, and Expanding the Genetic Spectrum
Abstract
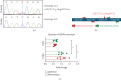
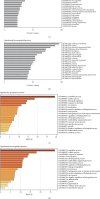
Rett syndrome (RTT) is a rare, X-linked, severe neurodevelopmental disorder, predominantly associated with pathogenic variants in the methyl-CpG-binding protein-2 (MECP2) gene, with an increasing number of atypical RTT or RTT-like individuals having pathogenic variants in other genes, such as cyclin-dependent kinase-like 5 (CDKL5) or forkhead box G1 (FOXG1). However, ~20% of individuals with a clinical diagnosis of RTT remain genetically undiagnosed, highlighting the importance of ongoing genomic and functional studies to expand the genetic spectrum of RTT. We present a female who was born to healthy nonconsanguineous parents and presented with severe intellectual disability, macrocephaly, ataxia, absent speech, and poor eye contact. The affected individual was clinically diagnosed with atypical RTT, but genetic testing showed no pathogenic variants in MECP2, CDKL5, or FOXG1. Singleton whole genome sequencing was conducted, which identified a heterozygous stop-gain variant [NM_001170629.2: c.5017C>T, p.(Arg1673∗)], in the chromodomain-helicase-DNA-binding protein 8 (CHD8) gene. Variant curation revealed its absence in unaffected populations, in silico predictions of pathogenicity, and an existing association with intellectual developmental disorder with autism and macrocephaly (IDDAM) (OMIM #615032). In vitro functional analyses, including Western blots, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and proteomic analyses, demonstrated a significant reduction of the CHD8 transcript and two CHD8 protein isoforms in the proband's skin fibroblasts relative to control fibroblasts. Additionally, proteomic analysis indicated a significant reduction of the MeCP2 protein, indicating a possible molecular link between CHD8 and MeCP2 and thus clinically between IDDAM and RTT. As the affected individual's phenotype is consistent with atypical RTT, our results suggest that CHD8 could be considered in the expanding genetic spectrum of atypical RTT, which may assist the diagnosis of other MECP2-negative RTT individuals.
Copyright © 2025 Elaine Zhang et al. Human Mutation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

