Utility of serum cytokine testing to differentiate complicated common variable immunodeficiency in resource limited settings
- PMID: 40497017
- PMCID: PMC12150043
- DOI: 10.1016/j.jacig.2025.100488
Utility of serum cytokine testing to differentiate complicated common variable immunodeficiency in resource limited settings
Abstract
Background: Common variable immunodeficiency (CVID) is the most common, symptomatic inborn error of immunity (IEI) worldwide. CVID diagnosis requires lymphocyte subset analysis by flow cytometry to delineate risk for autoimmune and inflammatory (AI) disease, known as complicated CVID. In resource-limited settings, reduced access to flow cytometry limits CVID diagnostics.
Objectives: We investigated the utility of serum cytokine testing, as compared to standard-of-care flow cytometry, for the diagnosis of AI disease in IEI and CVID.
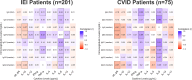
Methods: We performed a retrospective review of patients with International Classification of Diseases, Tenth Revision-coded IEI and extracted cytokine levels, tested on a clinically available serum-based multiplex assay of 13 parameters (soluble IL-2 receptor [sIL-2R], IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ). We assessed the association of cytokine levels with AI disease and immunophenotypes using Wilcoxon test or Spearman correlation, statistically adjusted for multiple testing. We compared predictive values of cytokine levels and lymphocyte subsets, measured by the area under the receiver-operating characteristic curve.
Results: In IEI and CVID, higher sIL-2R and IL-10 levels correlated with more AI complications per patient and more severe T-cell immunophenotypes. Composite receiver-operating characteristic curves for sIL-2R and IL-10, as compared to lymphocyte subsets (naive CD4+ T cells and class-switched memory B cells), had comparable diagnostic performance for AI disease in patients with IEI and CVID. These findings were validated in an independent IEI patient cohort.
Conclusions: sIL-2R and IL-10 testing had statistically comparable diagnostic performance for complicated CVID as compared to the current standard-of-care using flow cytometry.
Keywords: AI; CVID; CVIDc; Common variable immunodeficiency; IEI; IL-10; autoimmune and inflammatory; autoinflammation; complicated common variable immunodeficiency; cytokines; flow cytometry; inborn error of immunity; resource-limited care setting; soluble IL-2 receptor (sIL-2R); soluble biomarkers.
© 2025 The Author(s).
Conflict of interest statement
J.R.F. and M.S.O. are supported by the 10.13039/100006545National Institute on Minority Health and Health Disparities of the National Institute of Health under Award 1R01MD017816-01. J.R.F. and S.B. are supported by Faculty Development Awards from the 10.13039/100005276American Academy of Allergy, Asthma, & Immunology. S.B. is supported by the 10.13039/100000060National Institute of Allergy and Infectious Diseases of the 10.13039/100000002National Institutes of Health under Award Number K23AI163350. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Disclosure of potential conflict of interest: J. R. Farmer is an ongoing consultant for Pharming and has received investigator-initiated research grants from Pfizer, Bristol Myers Squibb, and Pharming with no direct relation to the work presented. S. Barmettler has been a consultant for CSL Behring and Octapharma and has received investigator-initiated research grants from Bristol Myers Squibb with no direct relation to the work presented. The rest of the authors declare that they have no relevant conflicts of interest.
Figures




References
-
- Gathmann B., Mahlaoui N., Gérard L., Oksenhendler E., Warnatz K., Schulze I., et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126.e11. - PubMed
-
- Seidel M.G., Kindle G., Gathmann B., Quinti I., Buckland M., van Montfrans J., et al. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. 2019;7:1763–1770. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

