First-in-class intranasal epinephrine spray for anaphylaxis: Dose finding clinical study
- PMID: 40497018
- PMCID: PMC12151664
- DOI: 10.1016/j.jacig.2025.100487
First-in-class intranasal epinephrine spray for anaphylaxis: Dose finding clinical study
Abstract
Background: Anaphylaxis is a life-threatening clinical presentation of acute systemic allergic reactions. Timely administration of epinephrine, usually by intramuscular autoinjector, is a robust life-saving treatment. Despite the critical necessity, there are multiple deterrents to patients' proper use of epinephrine autoinjectors. FMXIN002 is a novel nasal dry powder formulation of epinephrine in a single-use device, offering first-in-class alternative treatment.
Objectives: We sought to measure epinephrine pharmacokinetics, pharmacodynamics, and safety following a single administration of FMXIN002 at doses of 3.6 and 4.0 mg epinephrine versus intramuscular (IM) autoinjector 0.3 mg, in healthy adults.
Methods: This was an open-label, single-dose, 3-treatment, crossover, randomized, comparative bioavailability study with 12 healthy adults, female and male. FMXIN002 stability was also tested.
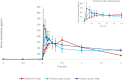
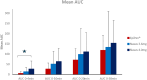
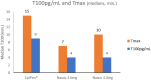
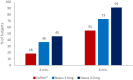
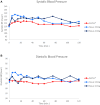
Results: FMXIN002 4.0 mg was absorbed faster and in higher amounts by most of the subjects, compared to IM autoinjector: 91% of subjects achieved the clinical threshold of 100 pg/mL plasma epinephrine at 6 minutes after administration of FMXIN002 4.0 mg compared to 55% of subjects treated with IM autoinjector. The area under the curve for 0 to 4 minutes' period was significantly higher for FMXIN002 4.0 mg (geometric mean: 7.49 h ∙ pg/mL vs 2.06 h ∙ pg/mL, respectively; P = .0377). The pharmacodynamic response and safety were comparable among all treatments. No serious adverse events occurred, all events were mild and self-resolved. FMXIN002 was highly stable at all tested conditions including 5 years at 20 ± 5ºC.
Conclusions: FMXIN002 4.0 mg nasal spray enables faster and higher epinephrine plasma absorbance at the short therapeutic window required for the treatment of anaphylaxis, using a patient-friendly, needle-free, stable and safe device.
Keywords: Anaphylaxis; allergy; bioavailability; epinephrine; intranasal; powder; spray.
© 2025 The Author(s).
Conflict of interest statement
The study was funded by Nasus Pharma, Israel. Disclosure of potential conflict of interest: D. Megiddo, C. Abrutzky, and T. Lapidot are employees of Nasus Pharma. Y. Tal is a consultant to Nasus Pharma. G.T. Krayz provides manufacturing and analytical services to Nasus Pharma. G. T. Krayz, D. Megiddo, C. Abrutzky, and T. Lapidot hold patents for the FMXIN002 nasal spray or are related to the patents assigned to Nasus Pharma. The rest of the authors declare that they have relevant conflicts of interest.
Figures


References
-
- Antolín-Amérigo D., Vidal-Albareda C., González de Olano D., de la Hoz-Caballer B. Current update on anaphylaxis: anaphylaxis management in recent guidelines. Eur Ann Allergy Clin Immunol. 2024;56:51–64. - PubMed
-
- Yost J., Brown E., Winders T., Jaffee H., Klein S., Martinez E., et al. Epinephrine autoinjector utilization and access in a nationally representative food-allergic adult sample. Ann Allergy Asthma Immunol. 2022;129(Suppl 5):S7.
LinkOut - more resources
Full Text Sources

